Realistic Cell Membranes
- Details
-
Last Updated: Thursday, 18 February 2021 16:53
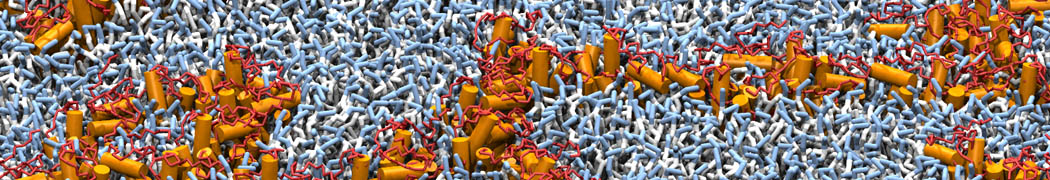
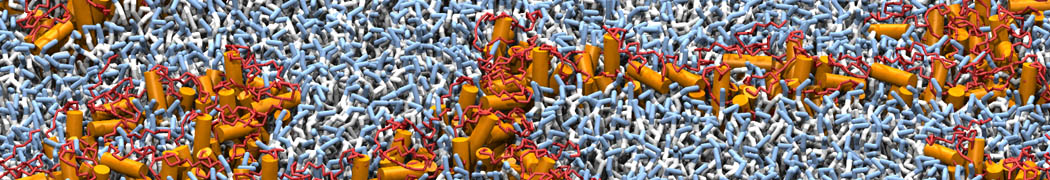
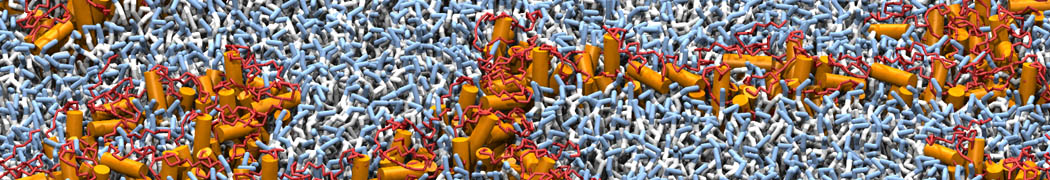
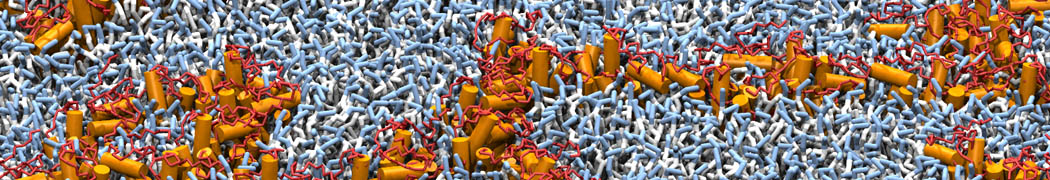
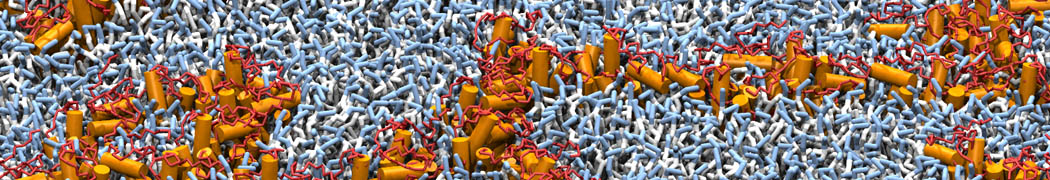
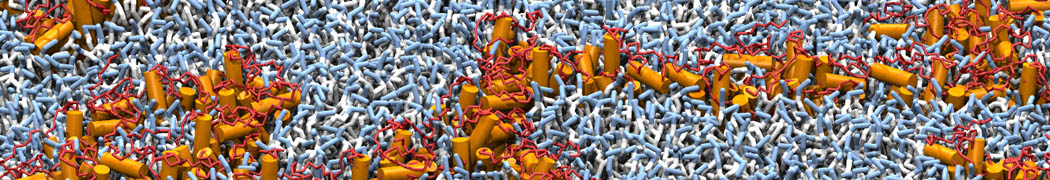
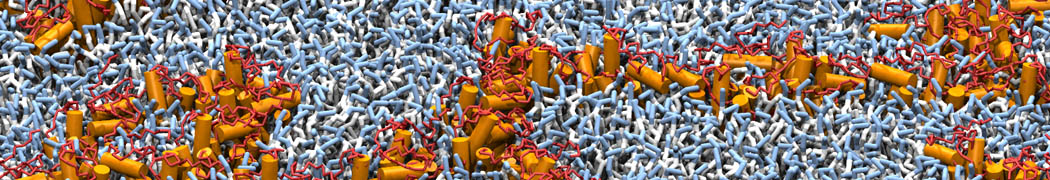
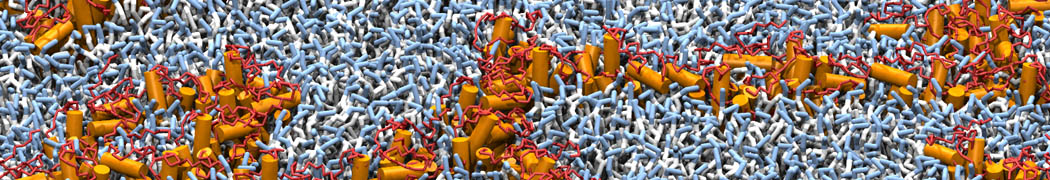
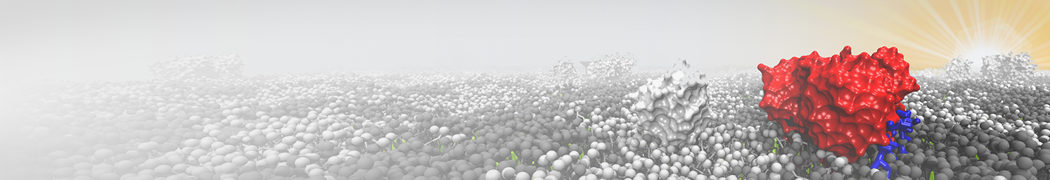
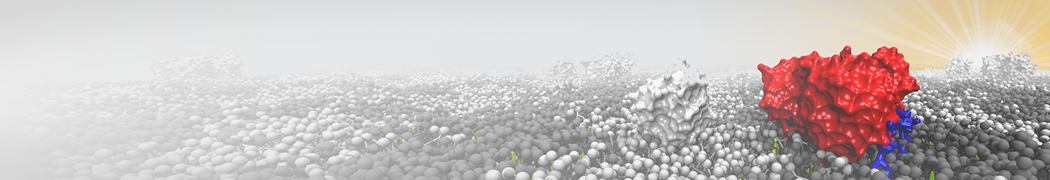
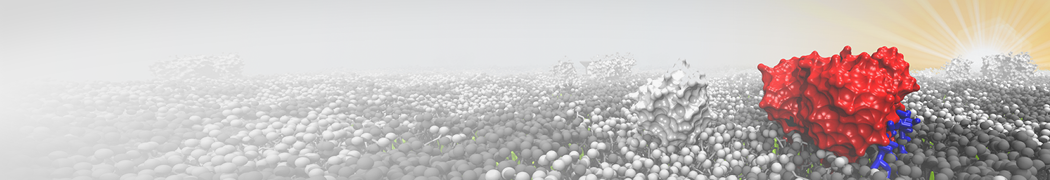
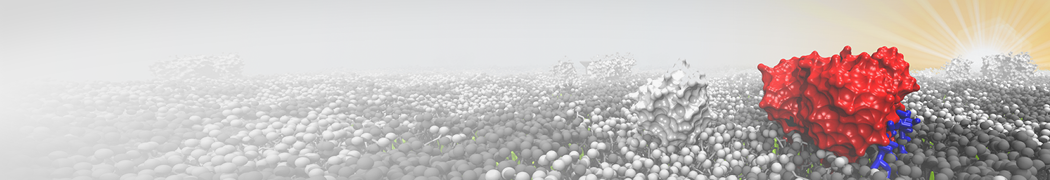
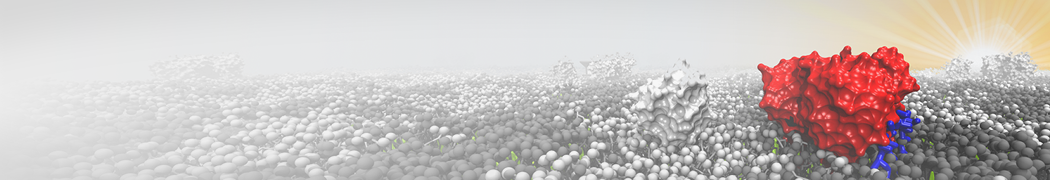
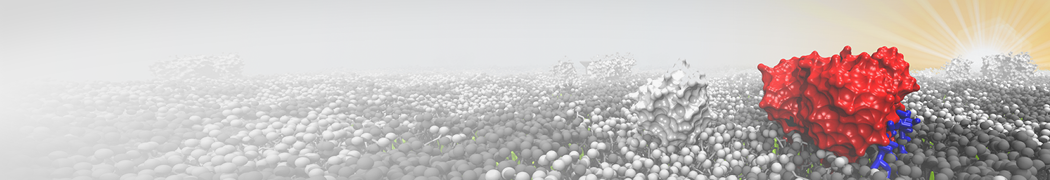
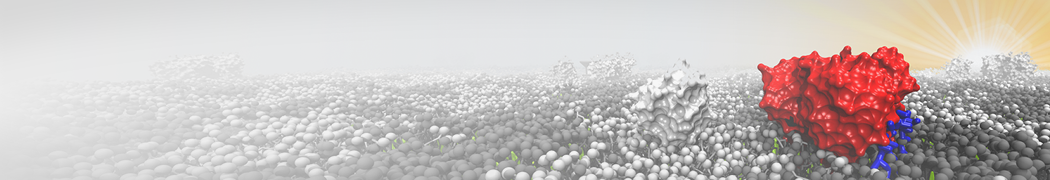
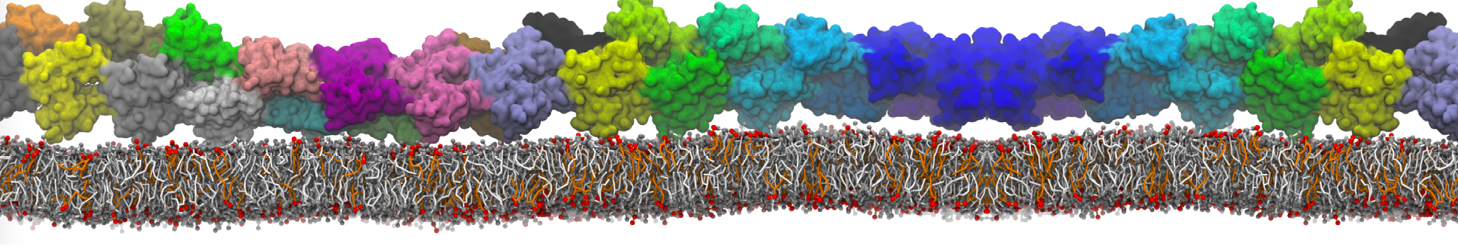
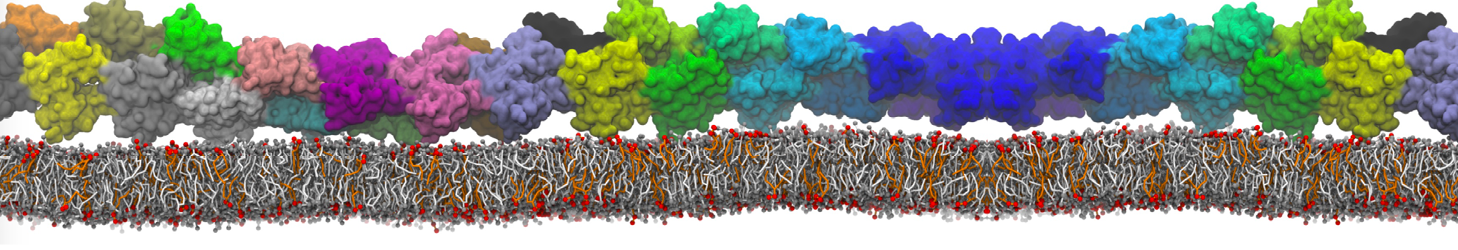
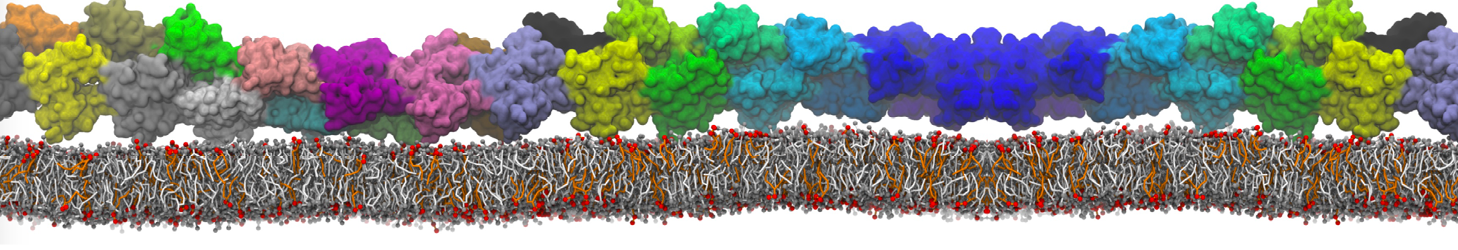
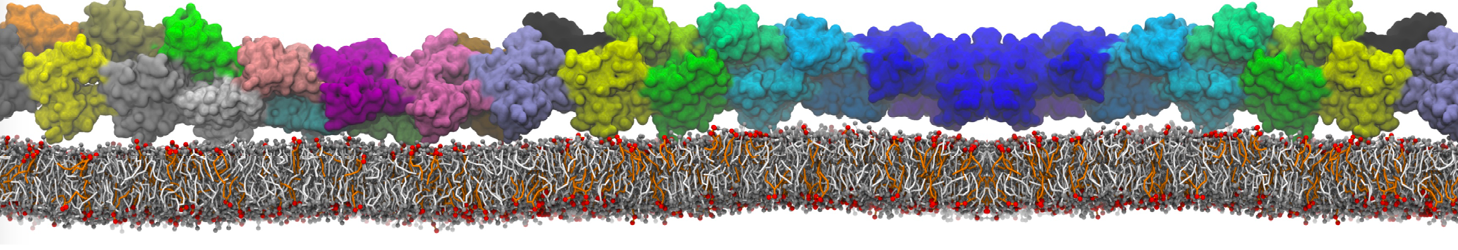
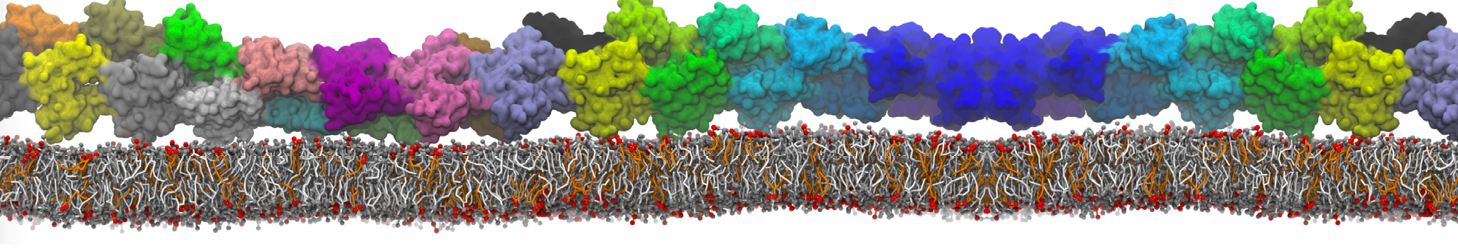
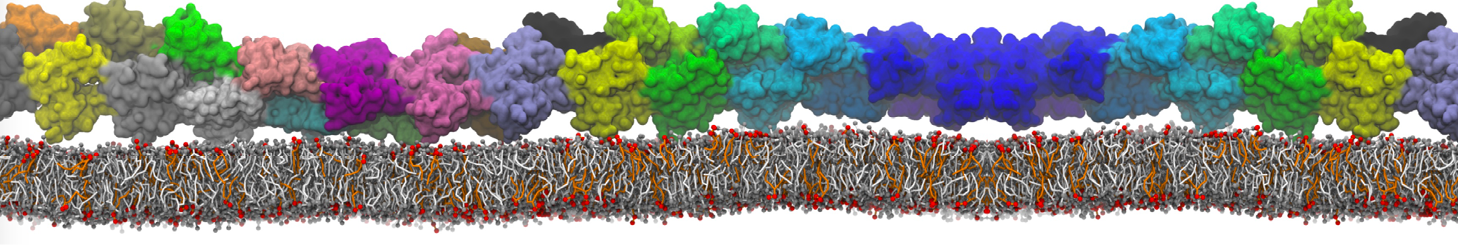
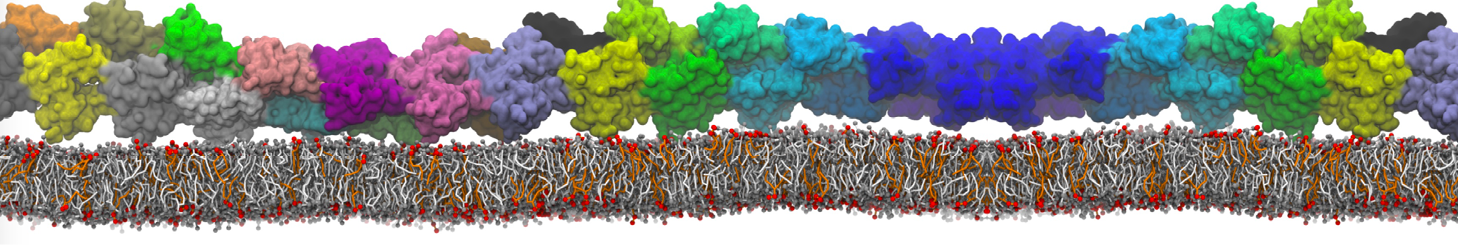
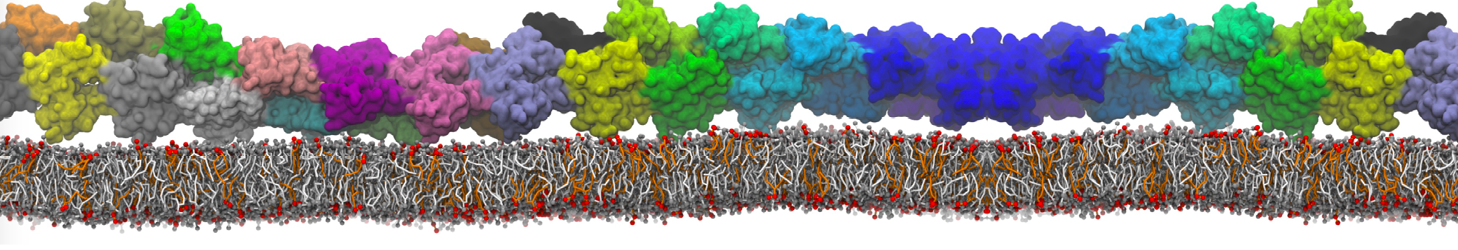
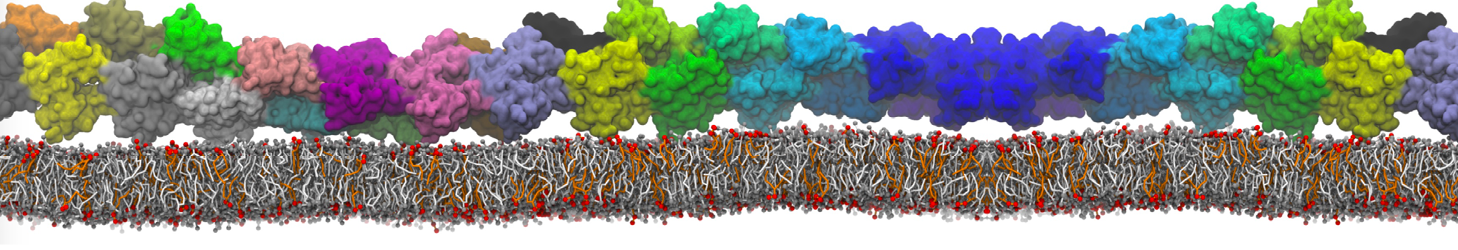
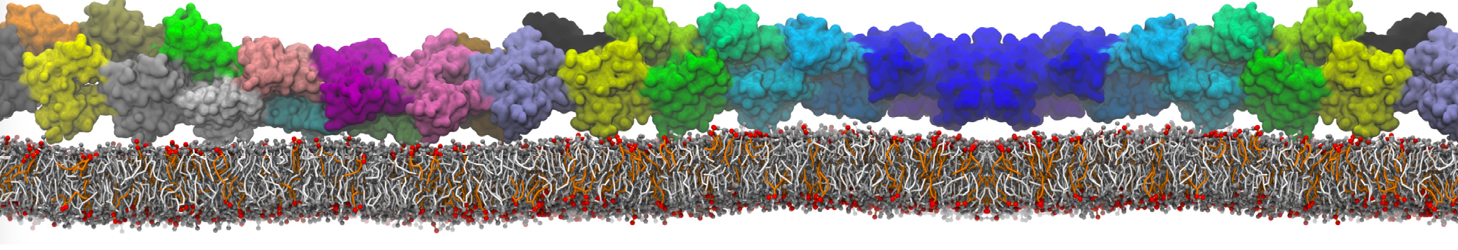
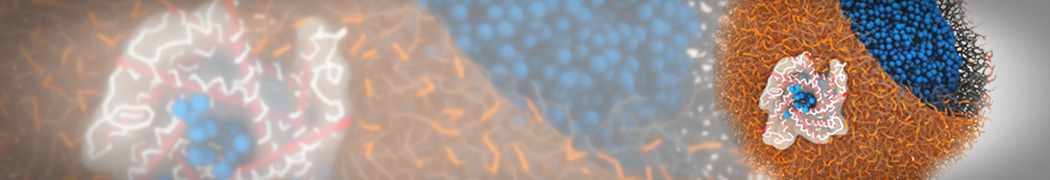
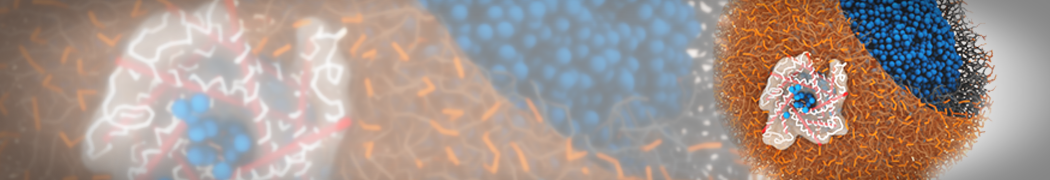
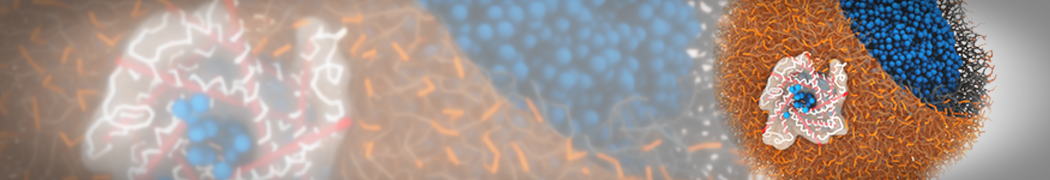
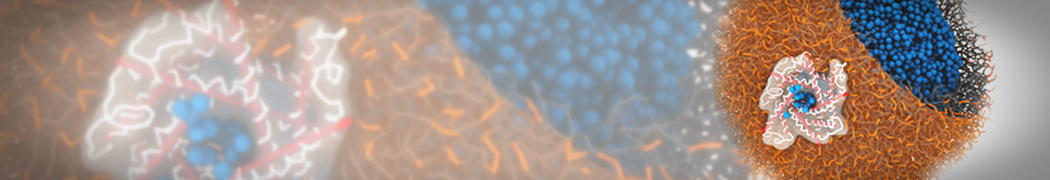
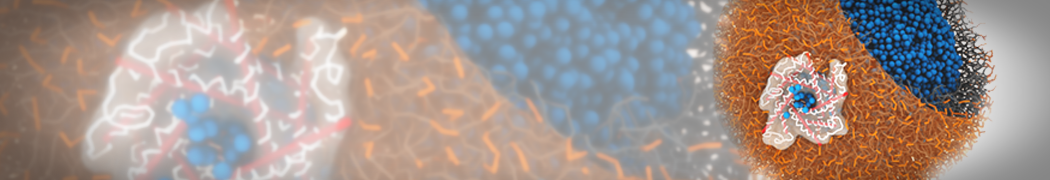
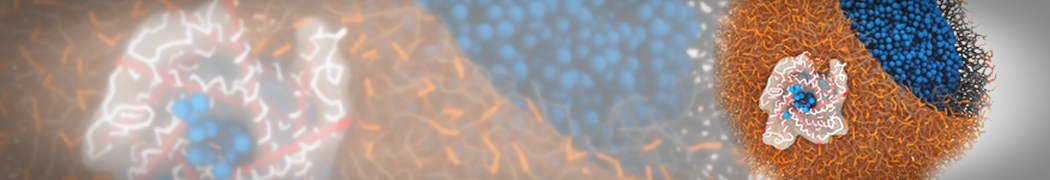
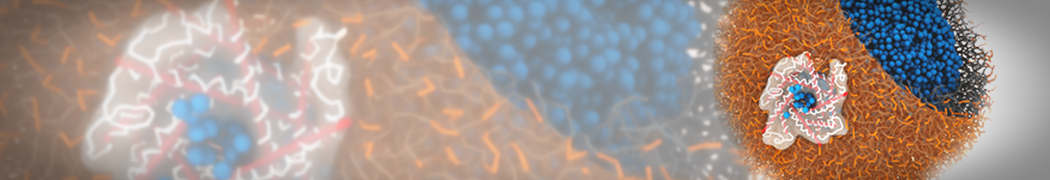
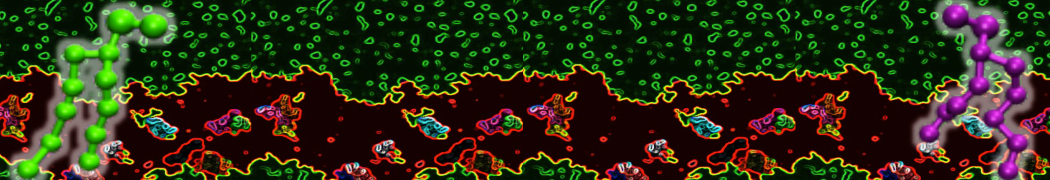
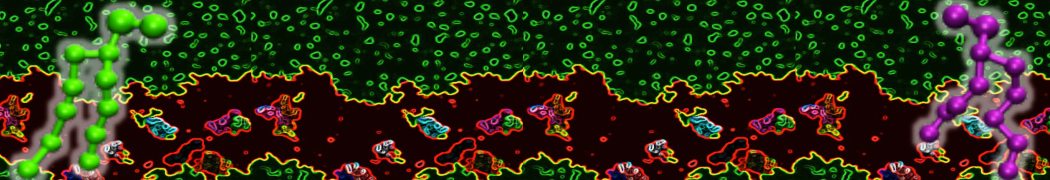
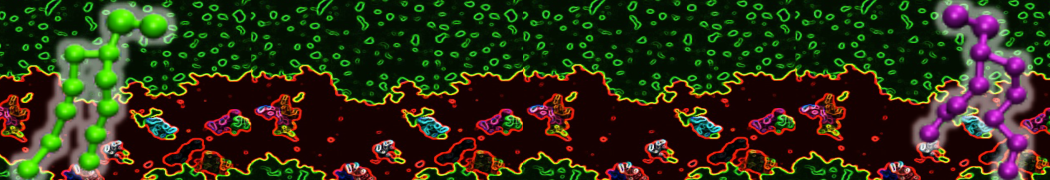
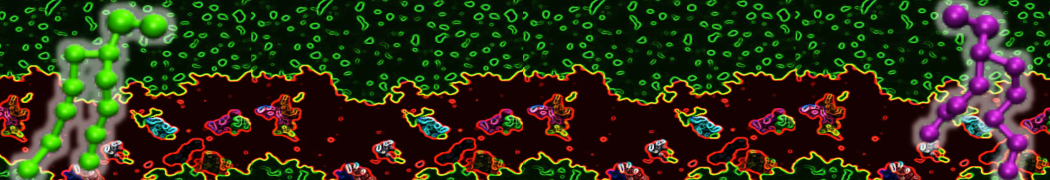
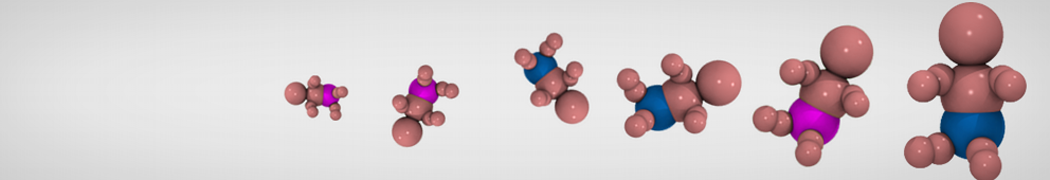
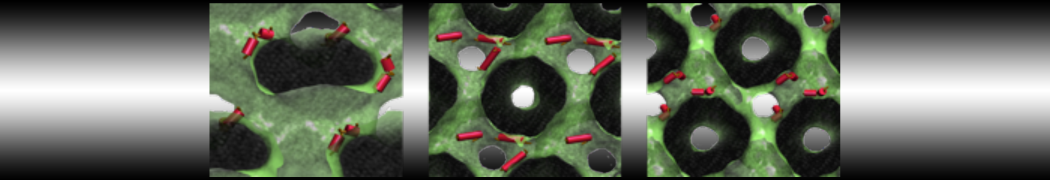
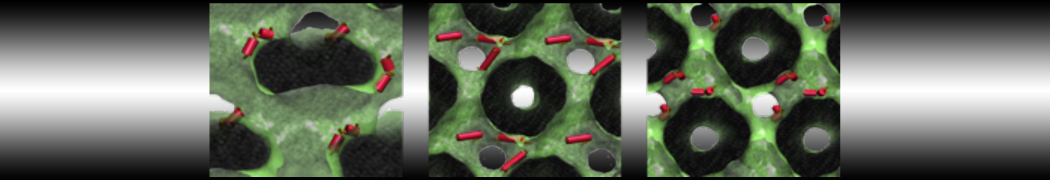
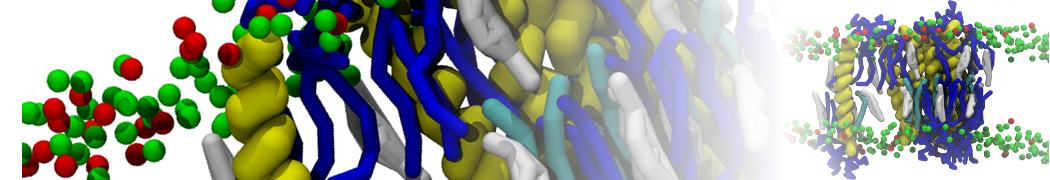
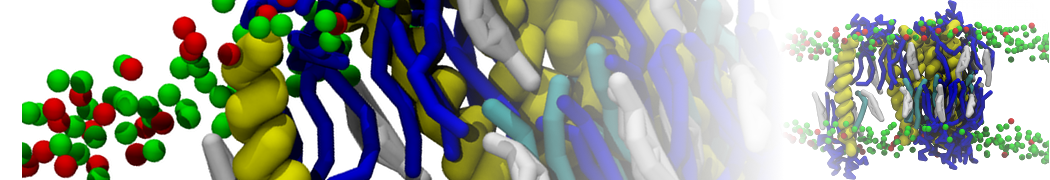
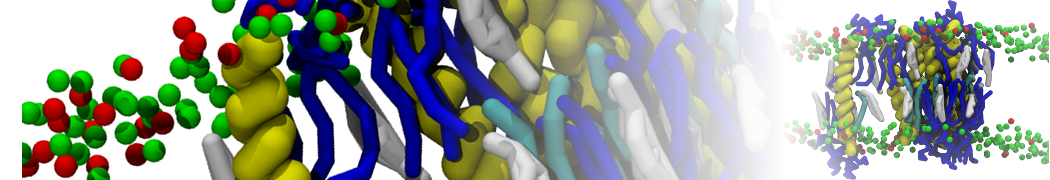
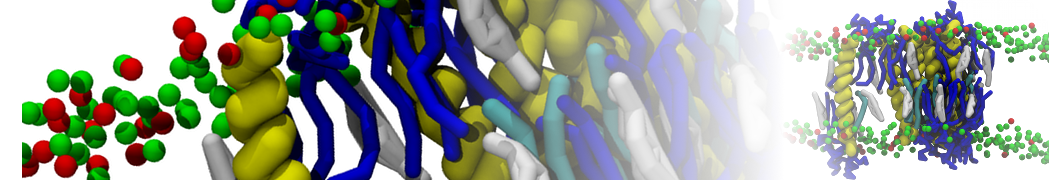
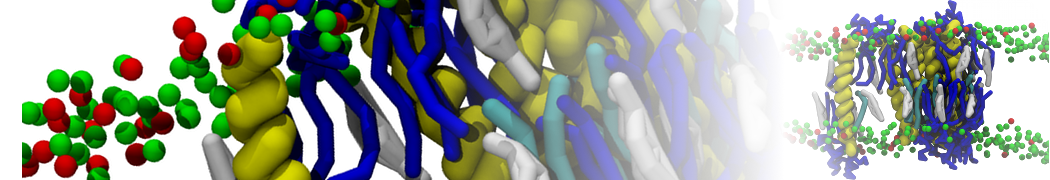
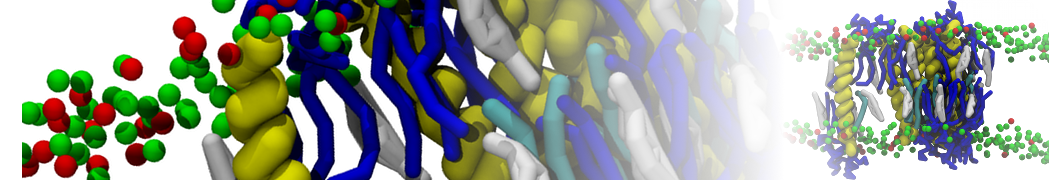
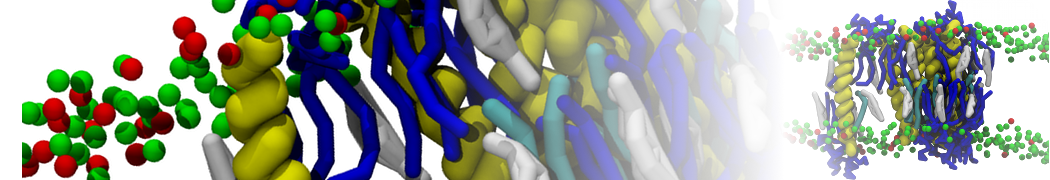
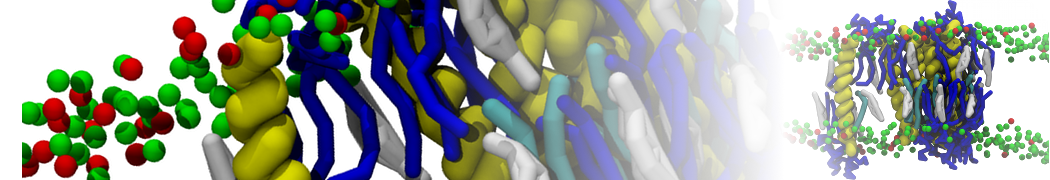
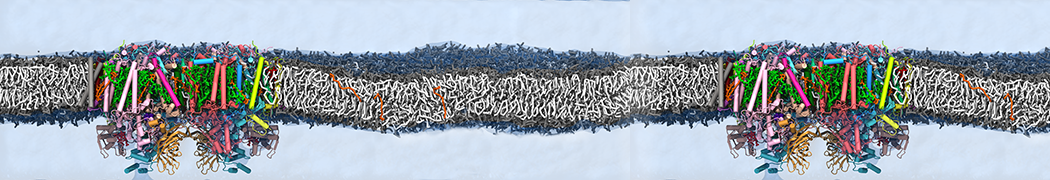
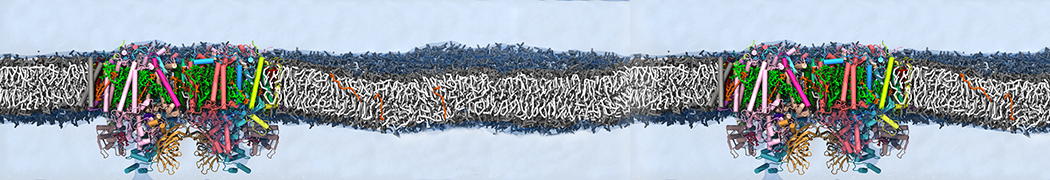
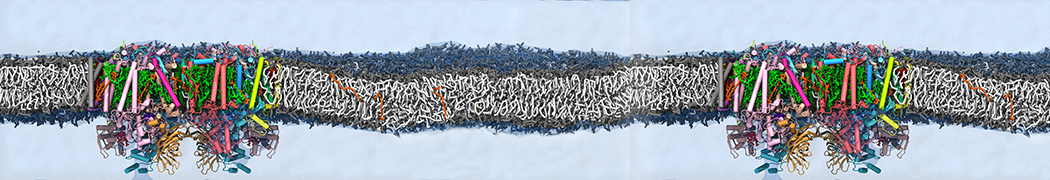
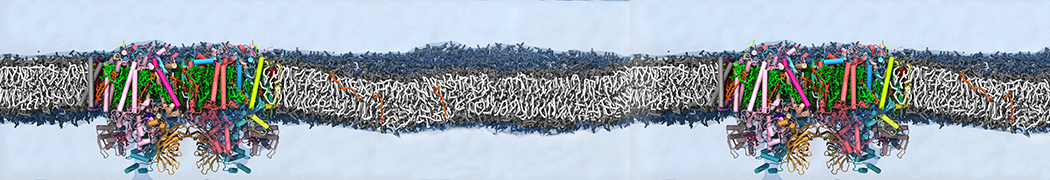
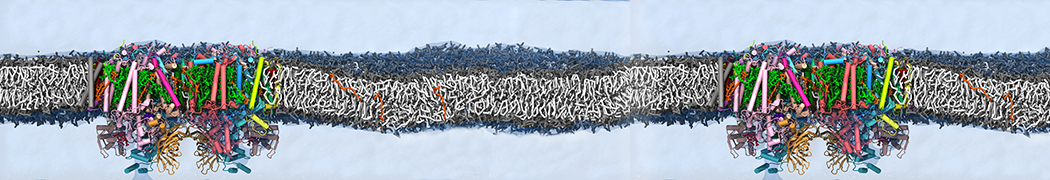
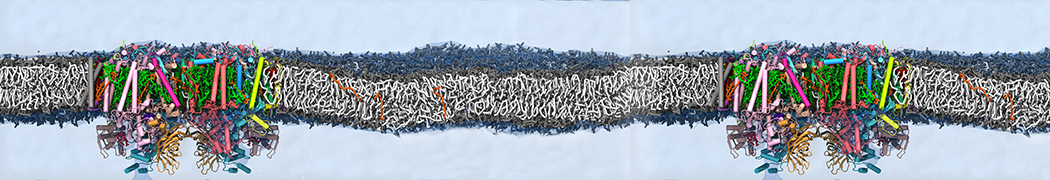
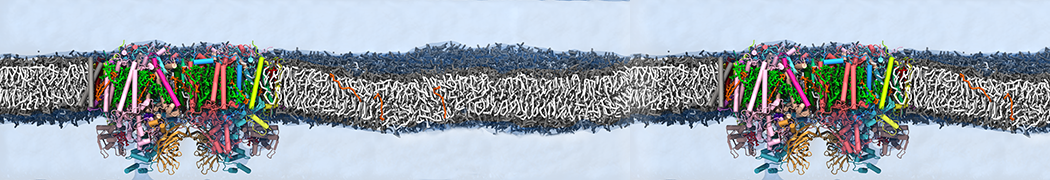
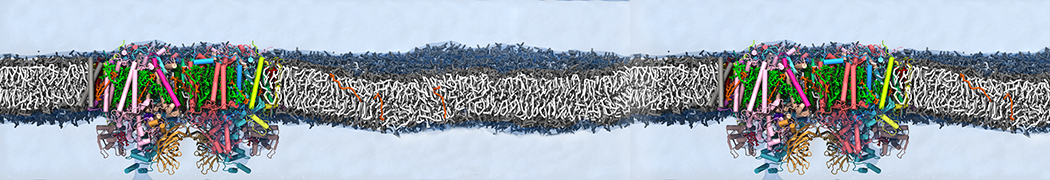
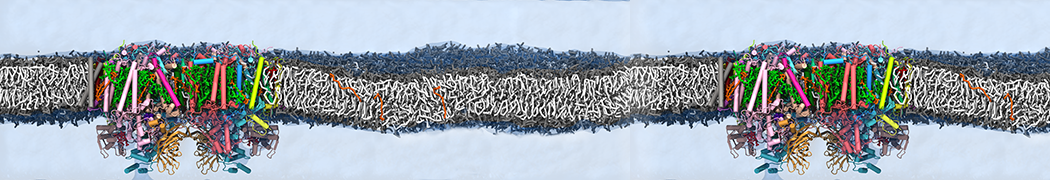
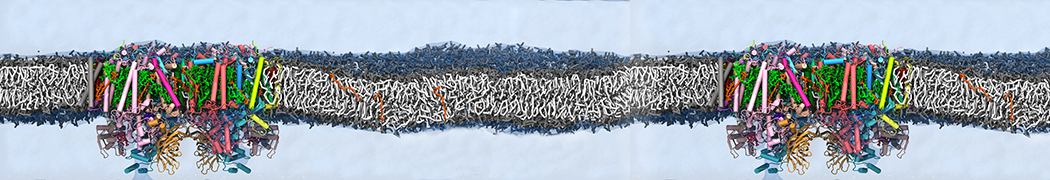
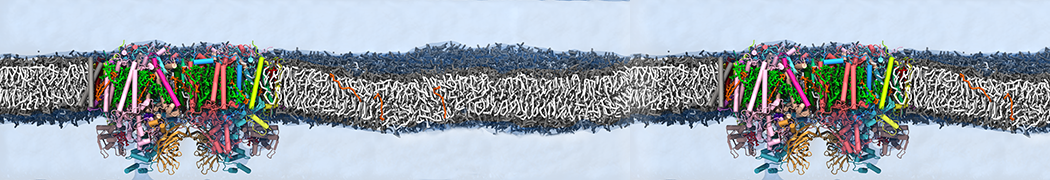
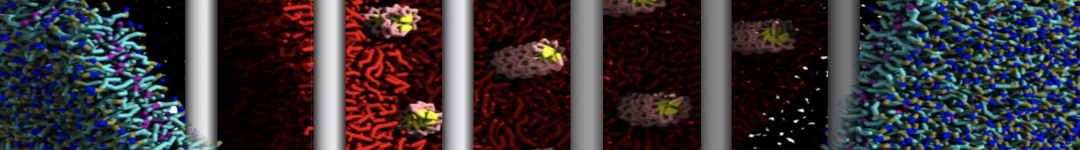
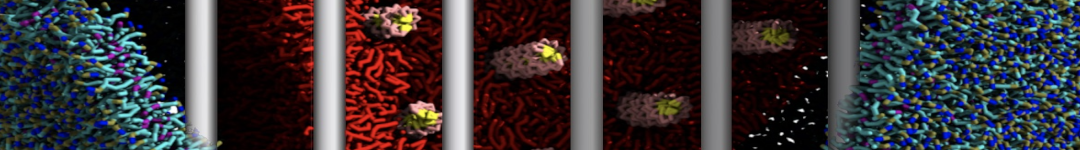
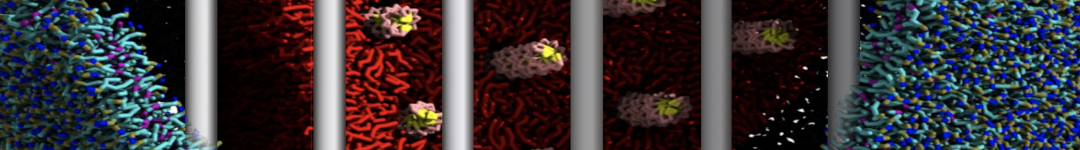
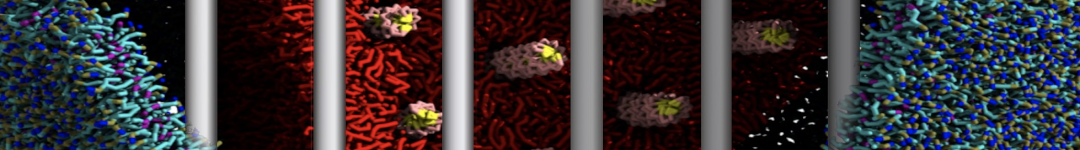
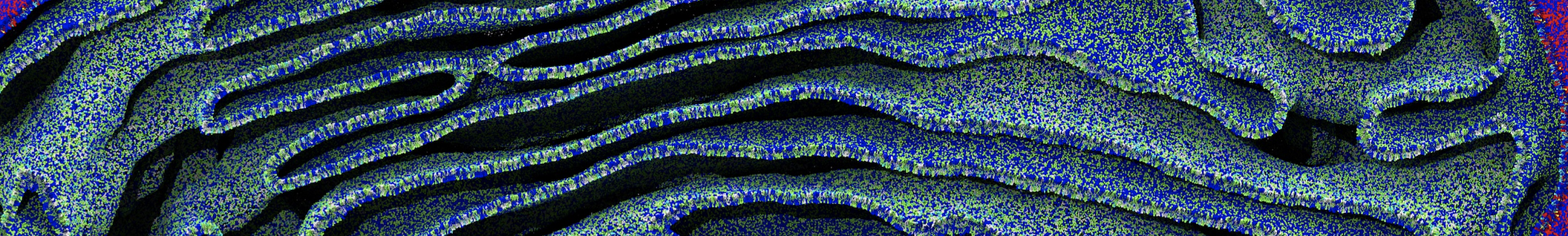
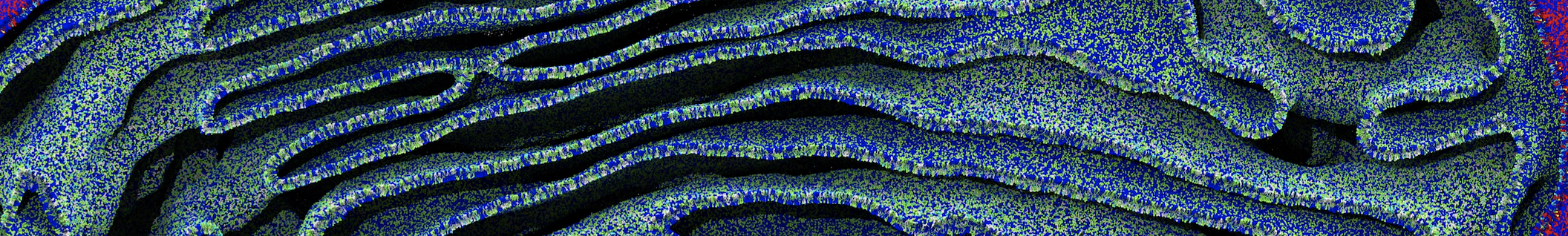
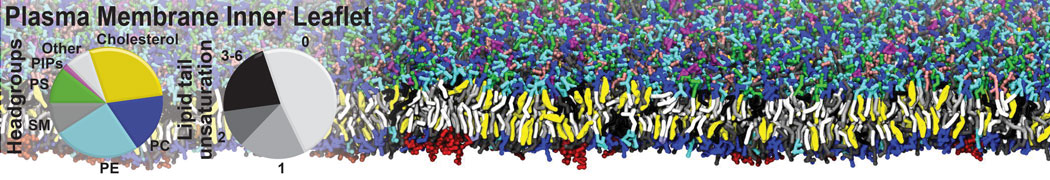
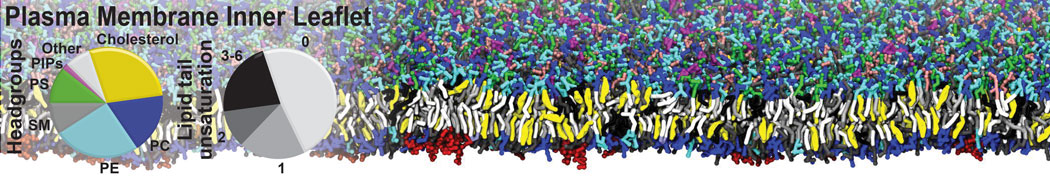
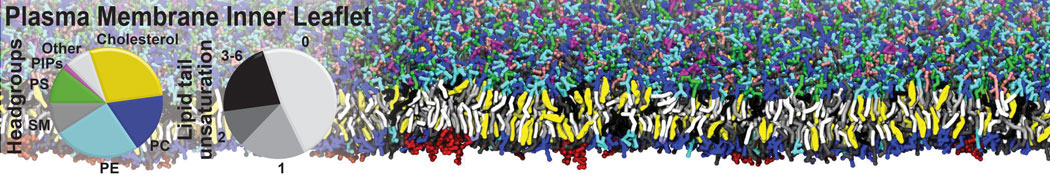
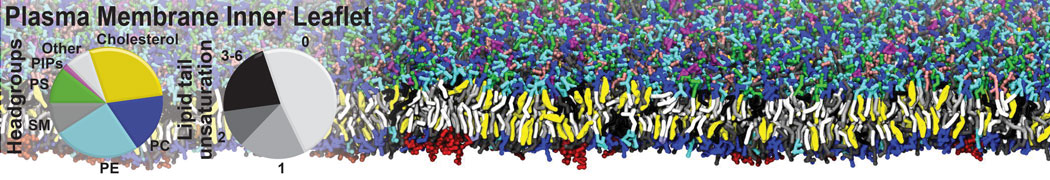
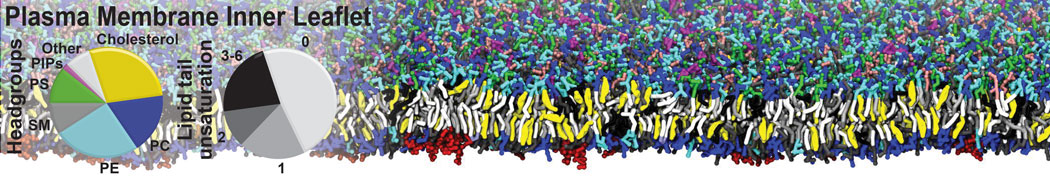
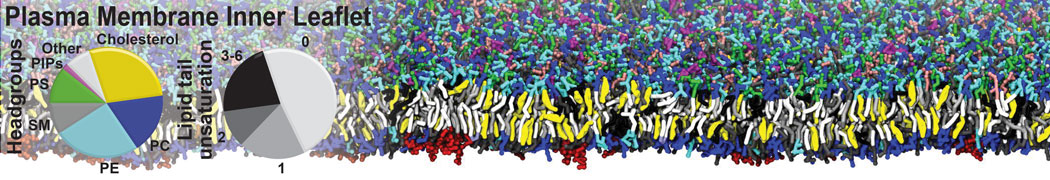
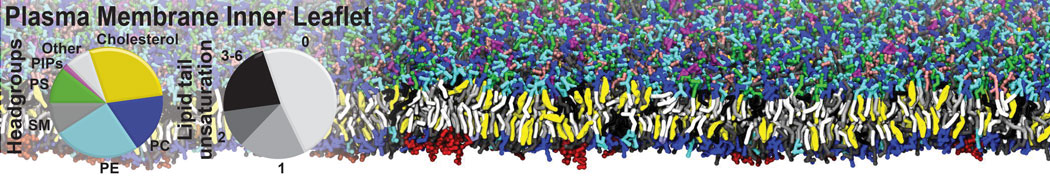
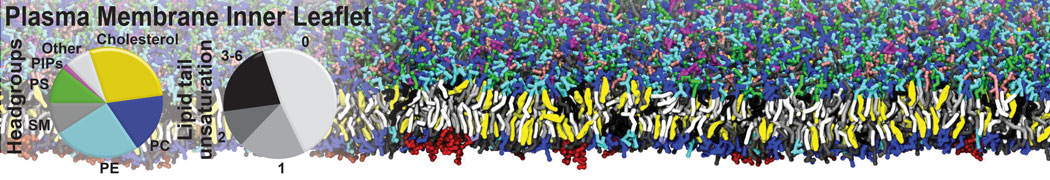
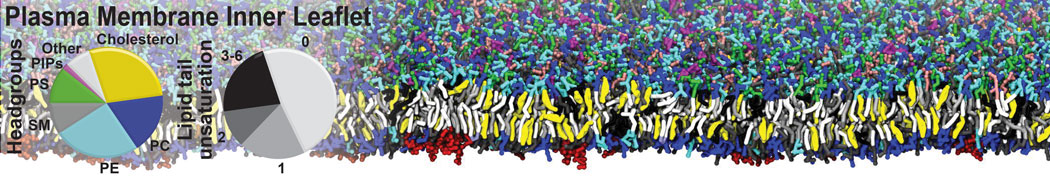
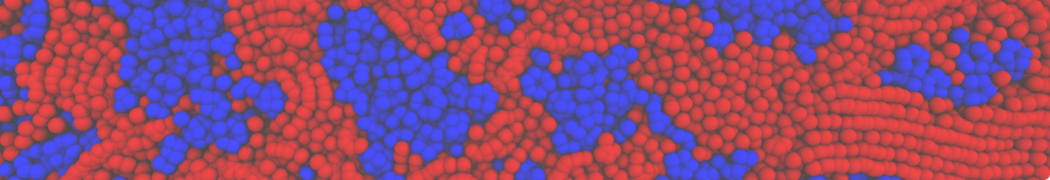
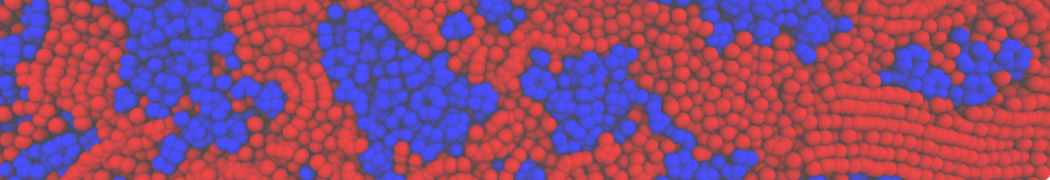
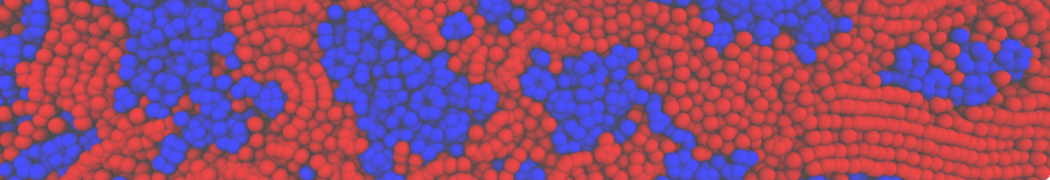
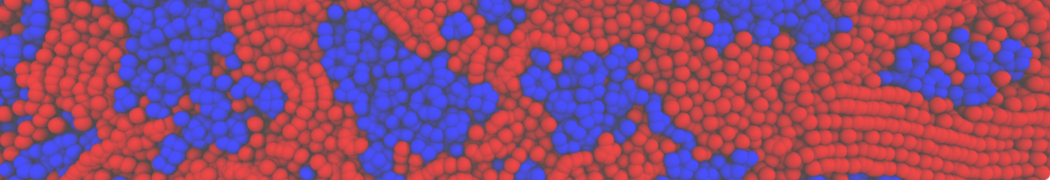
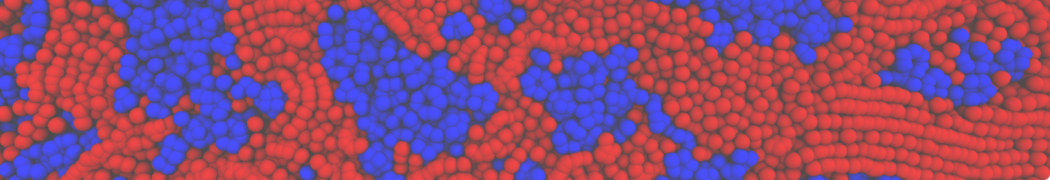
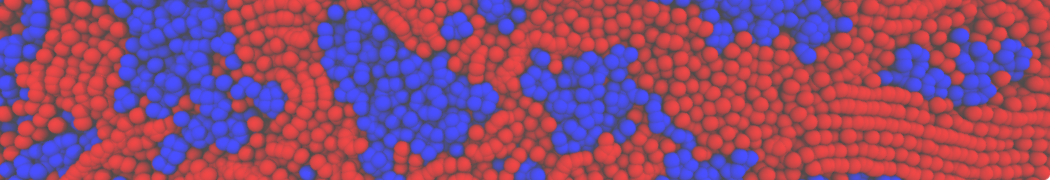
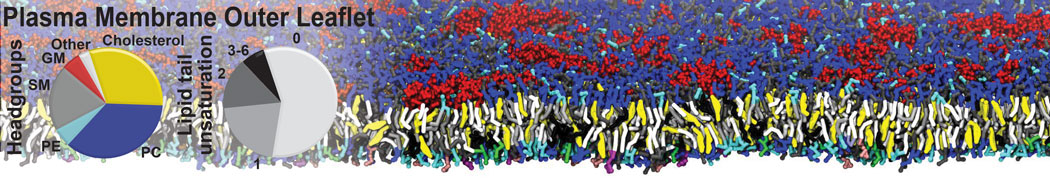
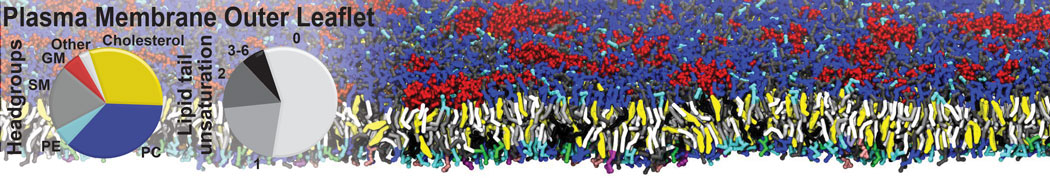
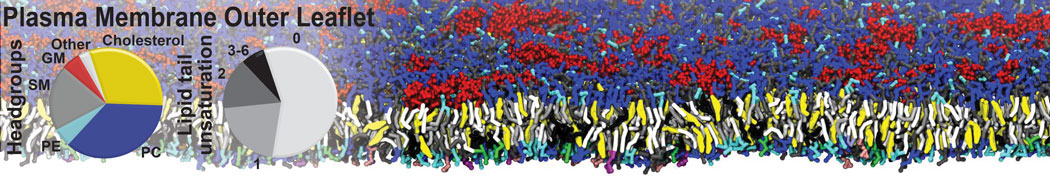
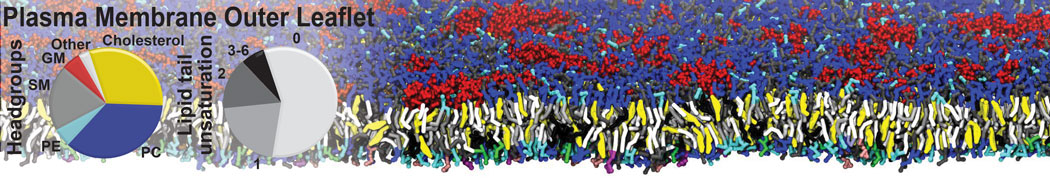
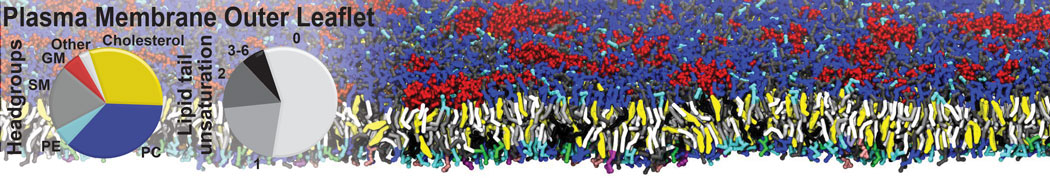
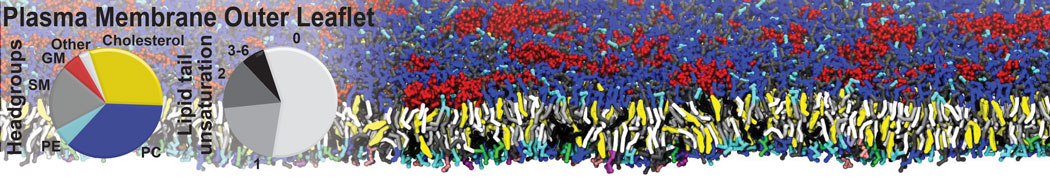
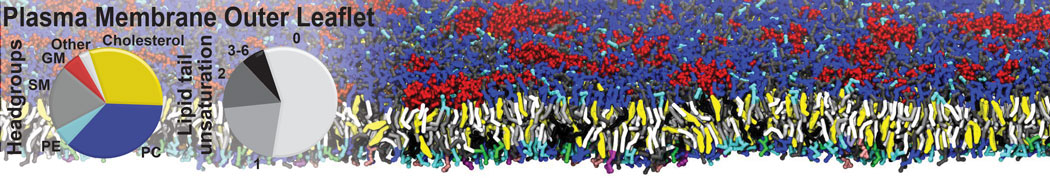
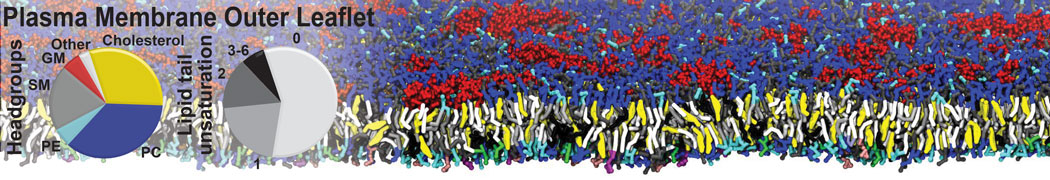
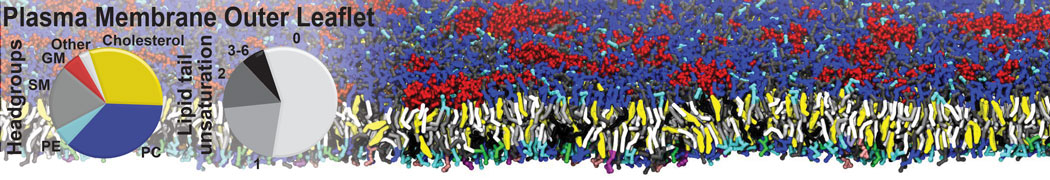
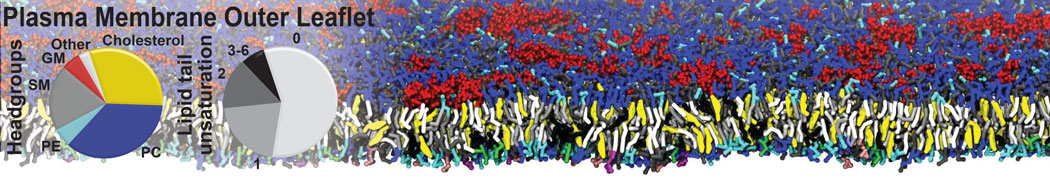
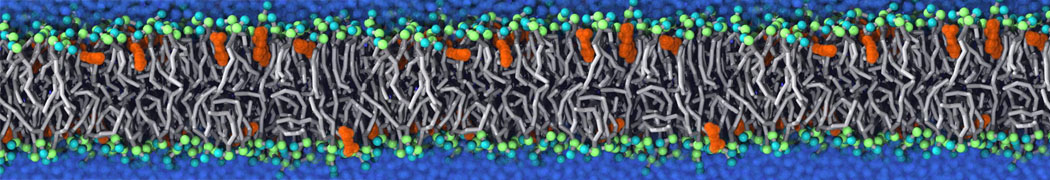
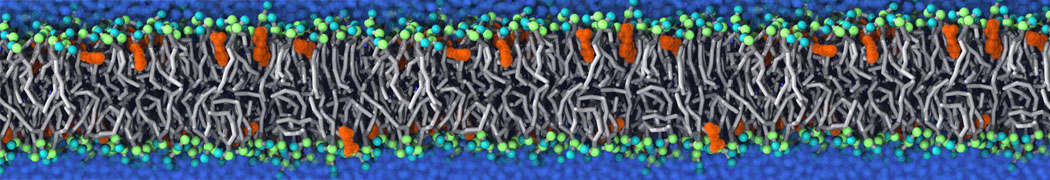
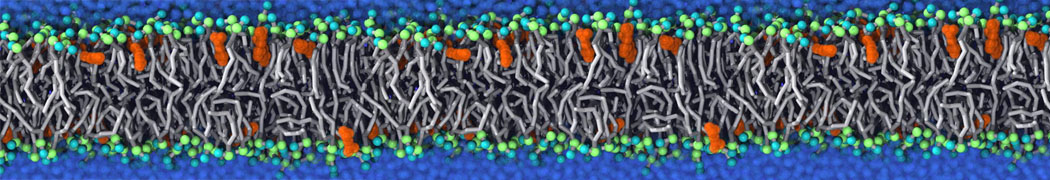
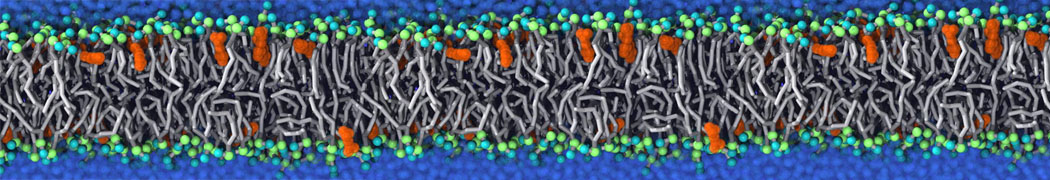
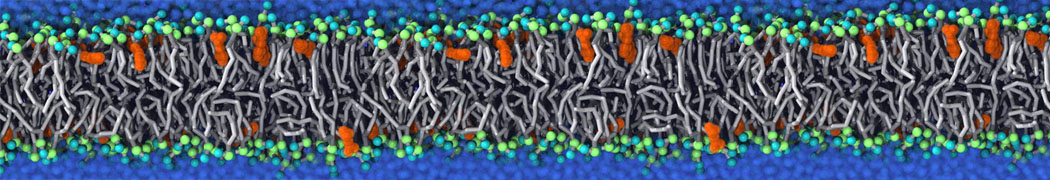
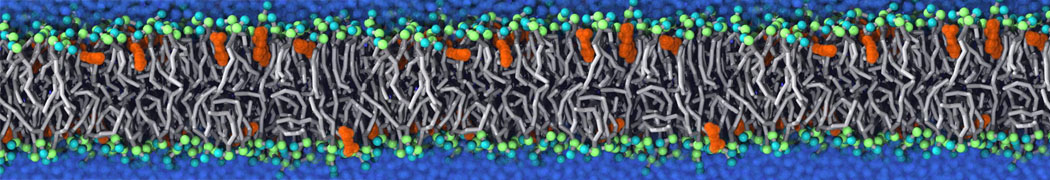
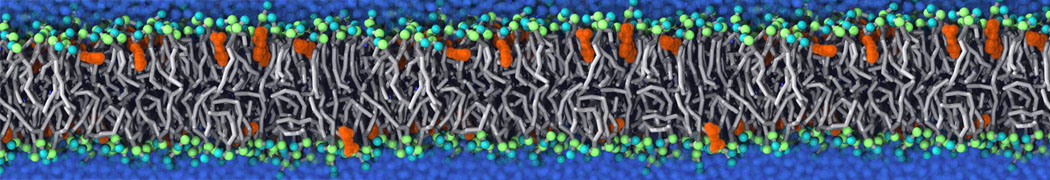
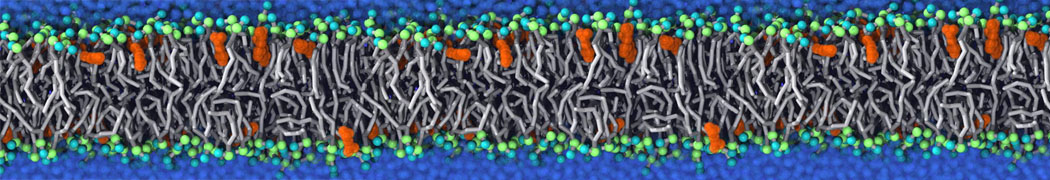
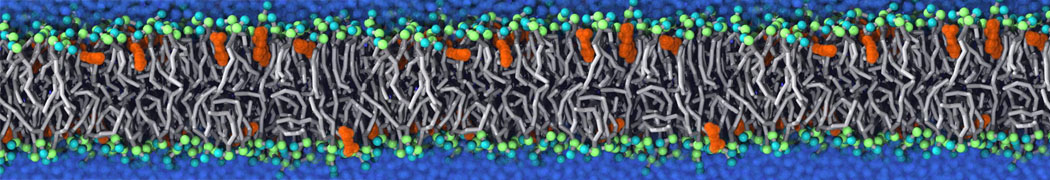
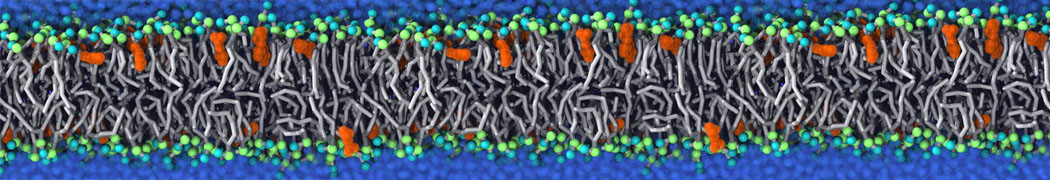
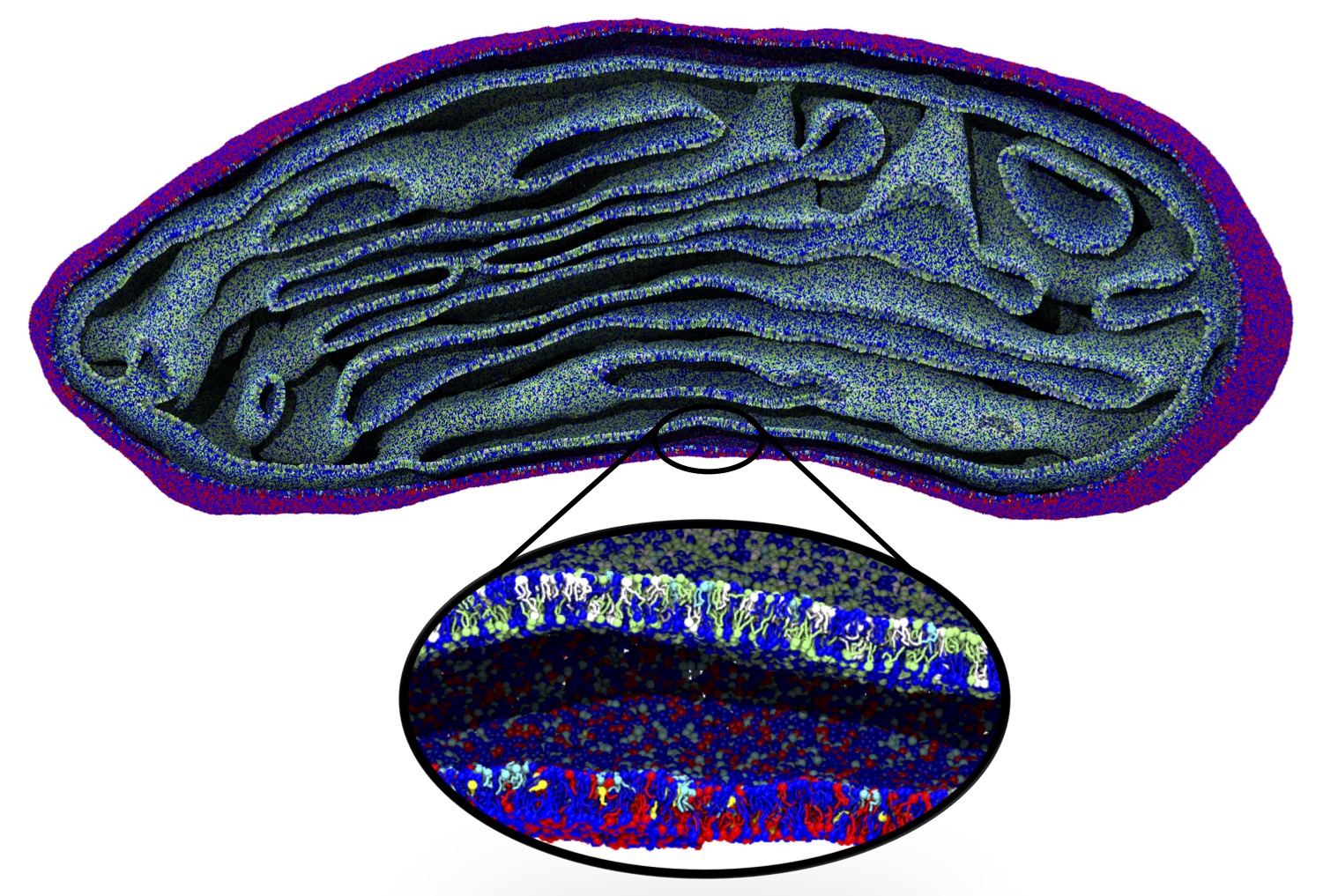 Biomembranes are essential cellular components. Together with membrane-adhered structures, such as the cytoskeleton, cell membranes constitute incredibly heterogeneous and crowded environments, containing hundreds of different lipid types and being densely packed with a large variety of membrane proteins. They provide identity not only to the cell as a whole, through the enveloping plasma membrane, but also to many internal organelles.
Biomembranes are essential cellular components. Together with membrane-adhered structures, such as the cytoskeleton, cell membranes constitute incredibly heterogeneous and crowded environments, containing hundreds of different lipid types and being densely packed with a large variety of membrane proteins. They provide identity not only to the cell as a whole, through the enveloping plasma membrane, but also to many internal organelles.
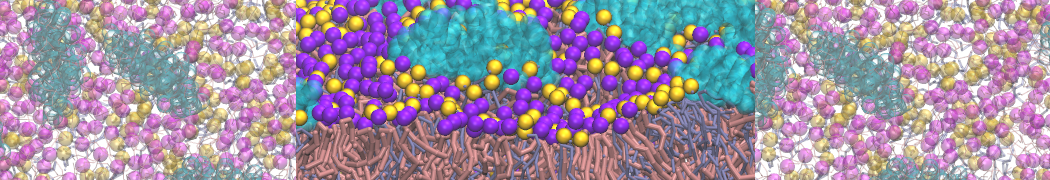
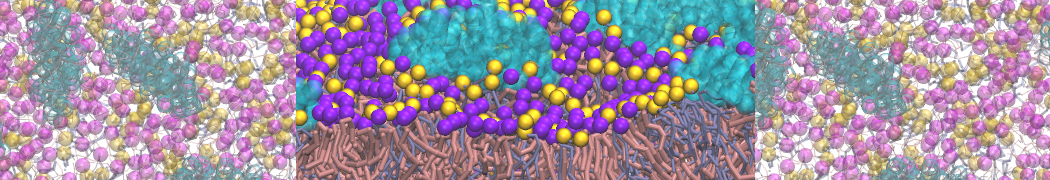
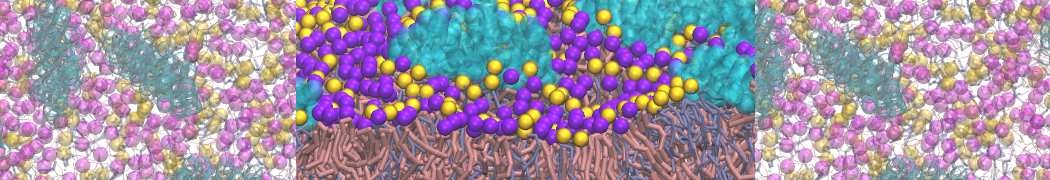
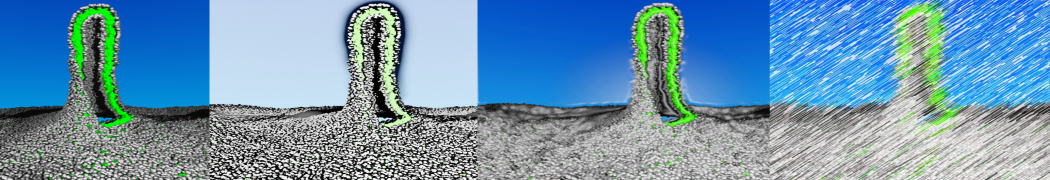
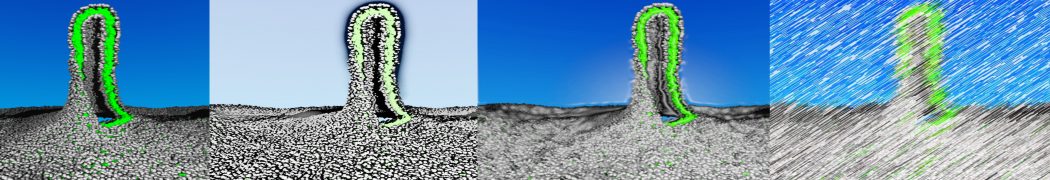
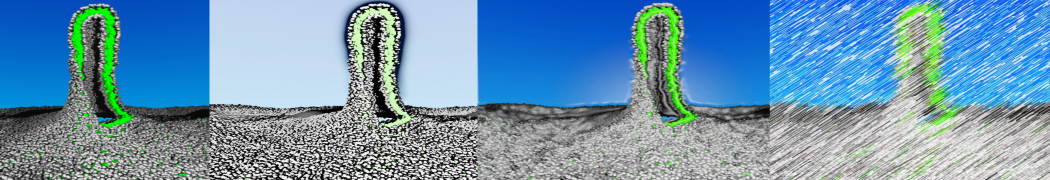
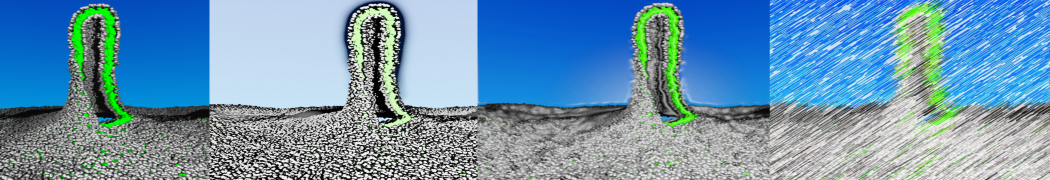
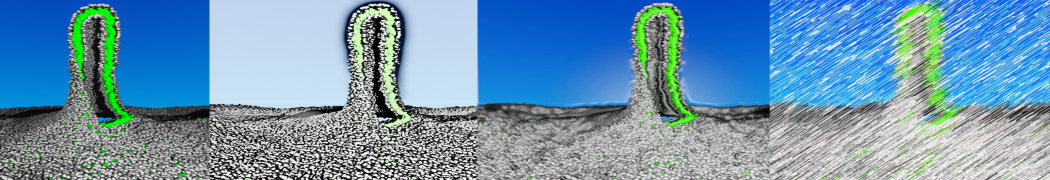
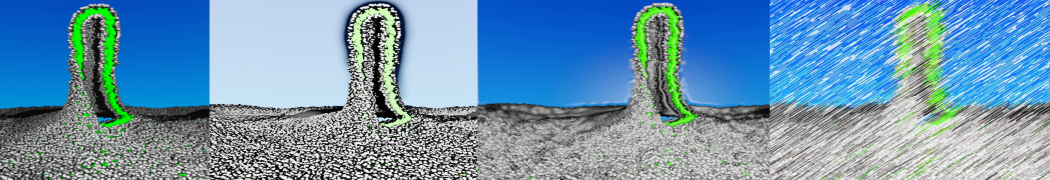
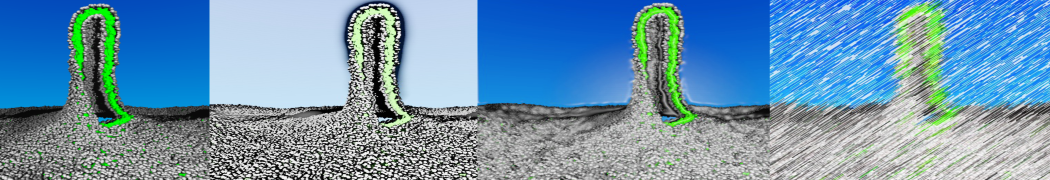
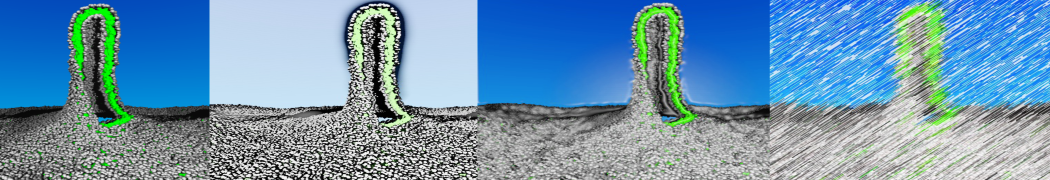
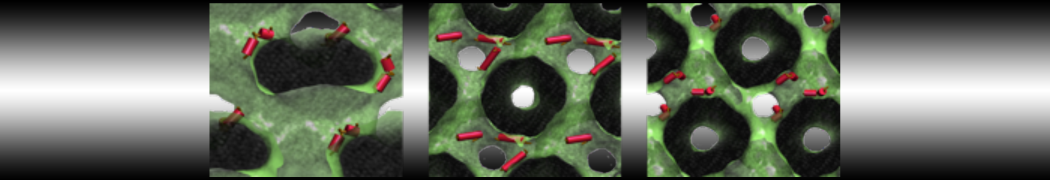
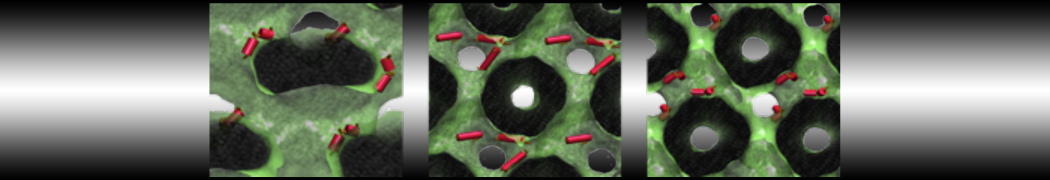
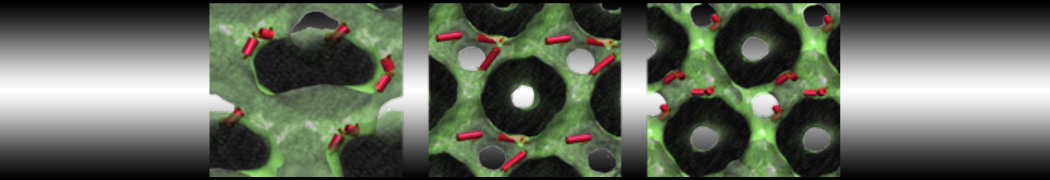
The Martini model is highly suited to capture the complexity of such cell membranes, as recently reviewed in [1]. Available tools such as Insane [2] and Charmm-GUI [3,4] facilitate setting up cell membranes with arbitrary complex compositions. Current highlights include the >60 lipid mixture representing mammalian plasma membranes [5-7], mixtures of galactolipids modeling the thylakoid membranes [8], the membranes of an enitre mitochondrion with realistic composition as well as shape [9, see Figure], and multi-component bacterial membranes [10].
The ability to simulate membranes with realistic lipid compositions is now enabling researchers to study the interaction of a variety of proteins and other compounds with such membranes, e.g. [11-14].
[1] S.J. Marrink, V. Corradi, P.C.T. Souza, H.I. Ingolfsson, D.P. Tieleman, M.S.P. Sansom. Computational Modeling of Realistic Cell Membranes. Chem. Review, 119:6184–6226, 2019. doi:10.1021/acs.chemrev.8b00460
[2] T.A. Wassenaar, H.I. Ingólfsson, R.A. Böckmann, D.P. Tieleman, S.J. Marrink. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. JCTC, 11:2144–2155, 2015. abstract
[3] Y. Qi, H.I. Ingólfsson, X. Cheng, J. Lee, S.J. Marrink, W. Im. CHARMM-GUI Martini Maker for coarse-grained simulations with the Martini force field. JCTC, 11:4486–4494, 2015. abstract
[4] P.C. Hsu, B.M.H. Bruininks, D. Jefferies, P.C. Telles de Souza, J. Lee, D.S. Patel, S.J .Marrink, Y. Qi, S. Khalid, W. Im. CHARMM‐GUI Martini Maker for modeling and simulation of complex bacterial membranes with lipopolysaccharides. J. Comput. Chem., 38:2354–2363, 2017. abstract
[5] H.I. Ingólfsson, M.N. Melo, F.J. van Eerden, C. Arnarez, C.A. López, T.A. Wassenaar, X. Periole, A.H. De Vries, D.P. Tieleman, S.J. Marrink. Lipid organization of the plasma membrane. JACS, 136:14554-14559, 2014. open access
[6] H.I. Ingólfsson, T.S. Carpenter, H. Bhatia, P.T. Bremer, S.J. Marrink, F.C. Lightstone. Computational Lipidomics of the Neuronal Plasma Membrane. Biophys. J. 113:2271–2280, 2017. open access
[7] S. Thallmair, H.I. Ingólfsson, S.J. Marrink. Cholesterol Flip-Flop Impacts Domain Registration in Plasma Membrane Models. J. Phys. Chem. Lett. 9:5527–5533, 2018. doi:10.1021/acs.jpclett.8b01877
[8] F.J. van Eerden, D.H. de Jong, A.H de Vries, T.A. Wassenaar, S.J. Marrink. Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. BBA Biomembranes, 1848:1319–1330, 2015. abstract
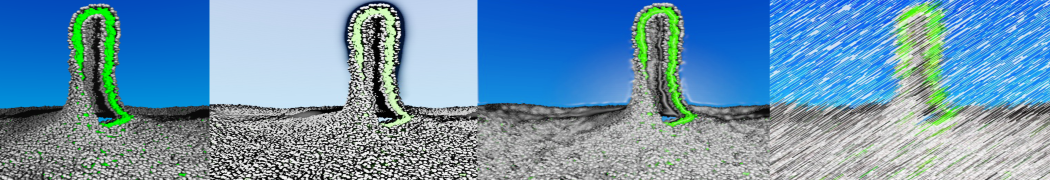
[9] W. Pezeshkian, M. Konig, T.A. Wassenaar, S.J. Marrink. Backmapping triangulated surfaces to coarse-grained membrane models. Nature Commun. 11:2296, 2020. doi.org/10.1038/s41467-020-16094-y
[10] P.C. Hsu, F. Samsudin, J. Shearer, S. Khalid. It Is Complicated: Curvature, Diffusion, and Lipid Sorting within the Two Membranes of Escherichia coli. JPC-Lett. 8 (22), 5513-5518, 2017.
[11] V. Corradi, E. Mendez-Villuendas, H.I. Ingólfsson, R.X. Gu, I. Siuda, M.N. Melo, A. Moussatova, L.J. DeGagné, B.I. Sejdiu, G. Singh, T.A. Wassenaar, K. Delgado Magnero, S.J. Marrink, D.P. Tieleman. Lipid–Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Central Science 4:709–717, 2018. doi:10.1021/acscentsci.8b00143
[12] S. Thallmair, P.A. Vainikka, S.J. Marrink. Lipid Fingerprints and Cofactor Dynamics of Light-Harvesting Complex II in Different Membranes. Biophys. J., 116:1446-1455, 2019. doi:10.1016/j.bpj.2019.03.009
[13] J. Shearer, D. Jefferies, S .Khalid. Outer membrane proteins OmpA, FhuA, OmpF, EstA, BtuB, and OmpX have unique lipopolysaccharide fingerprints. J. Chemical Theory and Computation 15 (4), 2608-2619, 2019.
[14] A. Buyan, C.D. Cox, J. Barnoud, J. Li, H.S.M. Chan, B. Martinac, S.J. Marrink, B. Corry. Piezo1 forms specific, functionally important interactions with phosphoinositides and cholesterol. Biophys. J. 119:1683-1697, 2020. doi.10.1016/j.bpj.2020.07.043
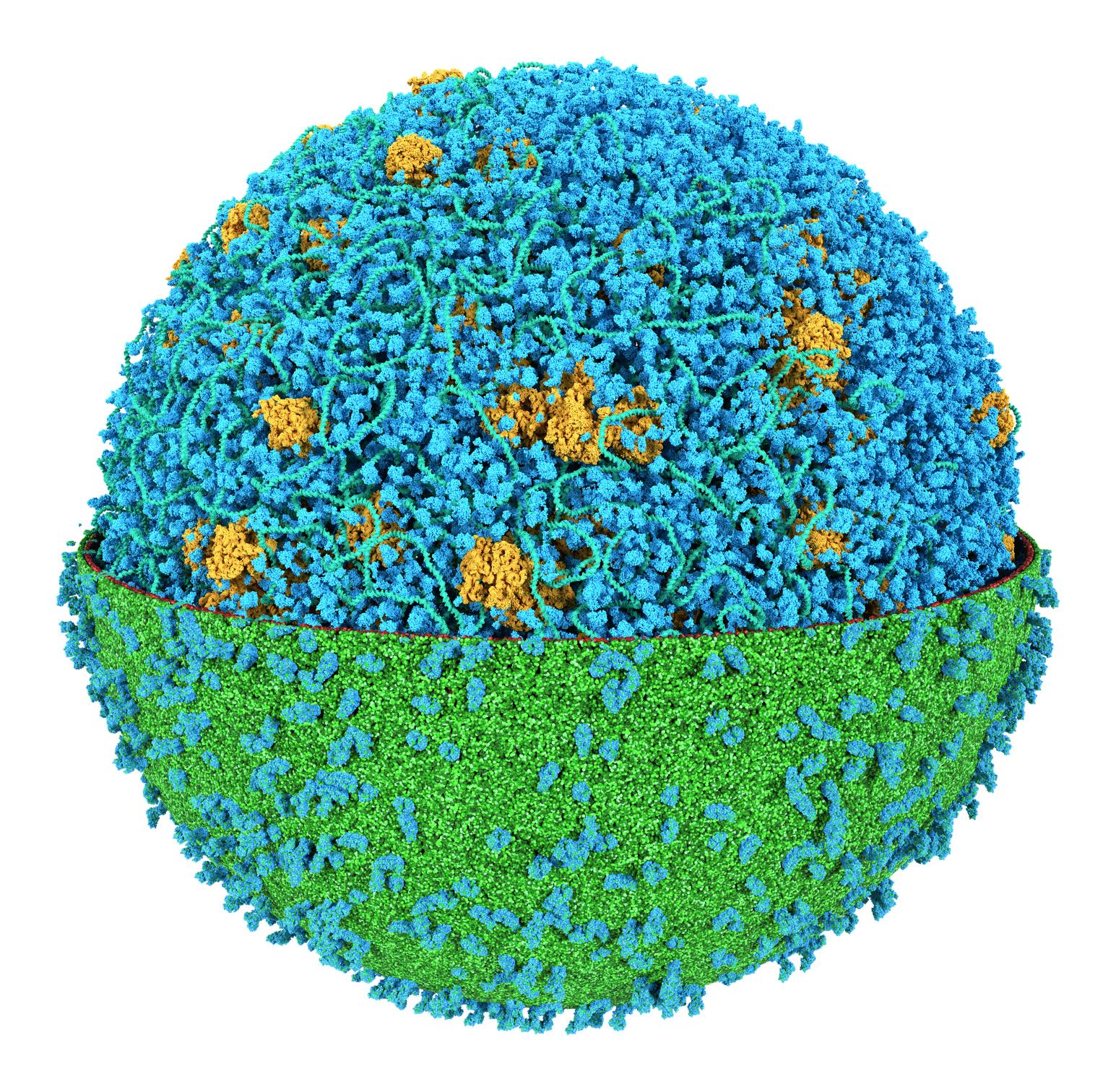

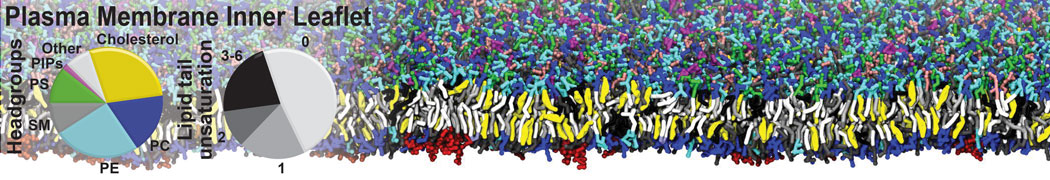
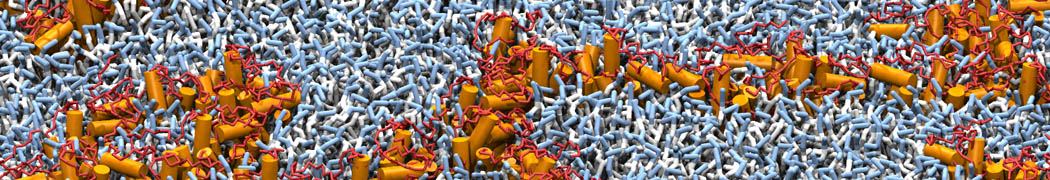
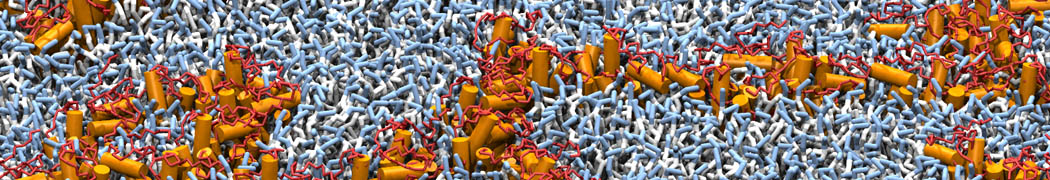
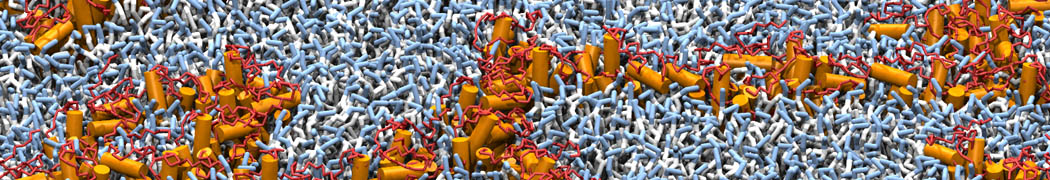
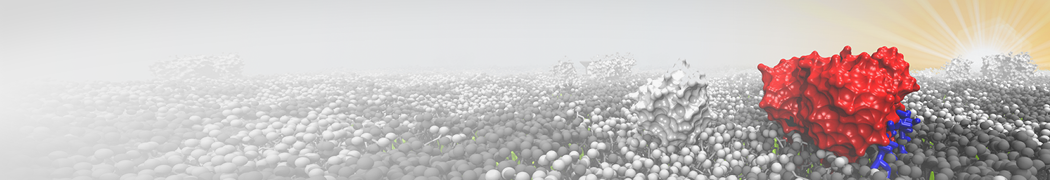
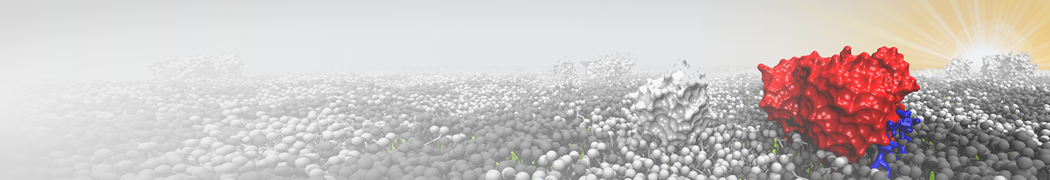
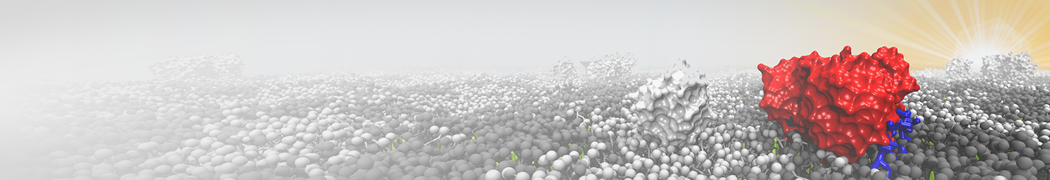
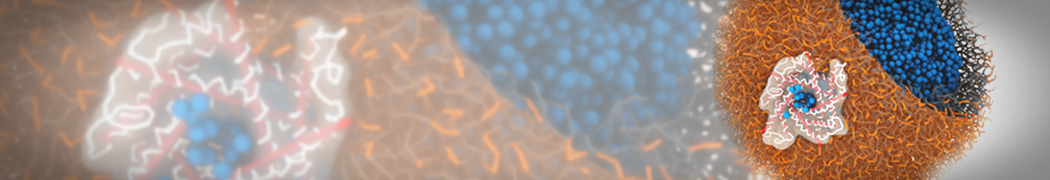
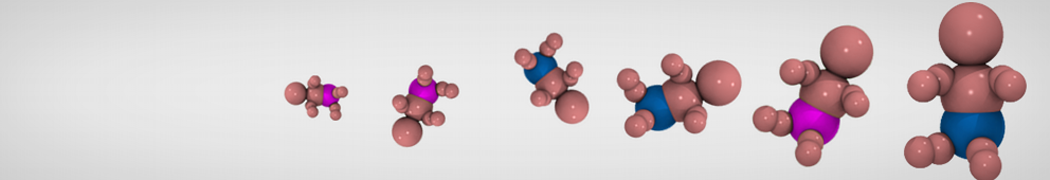
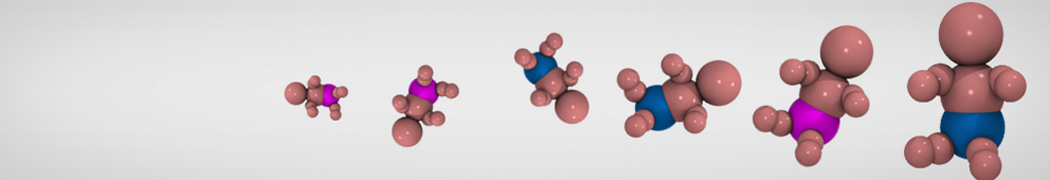
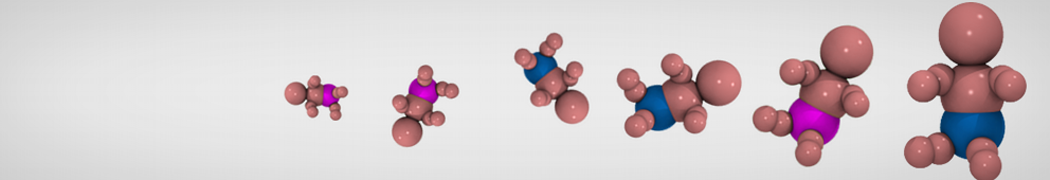
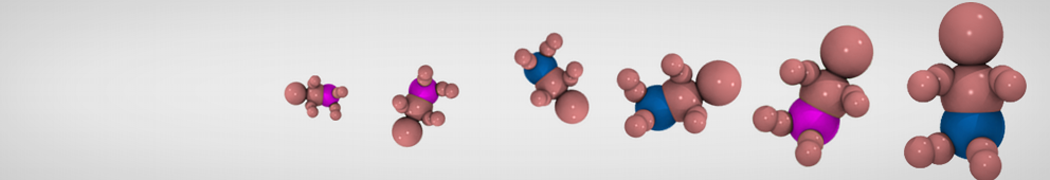
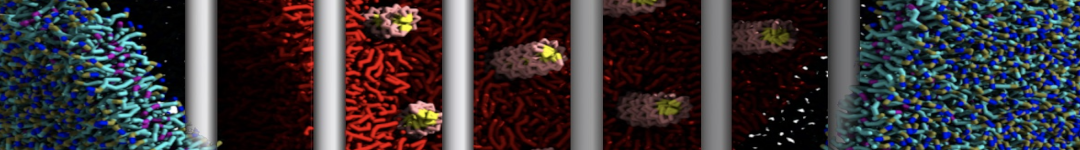


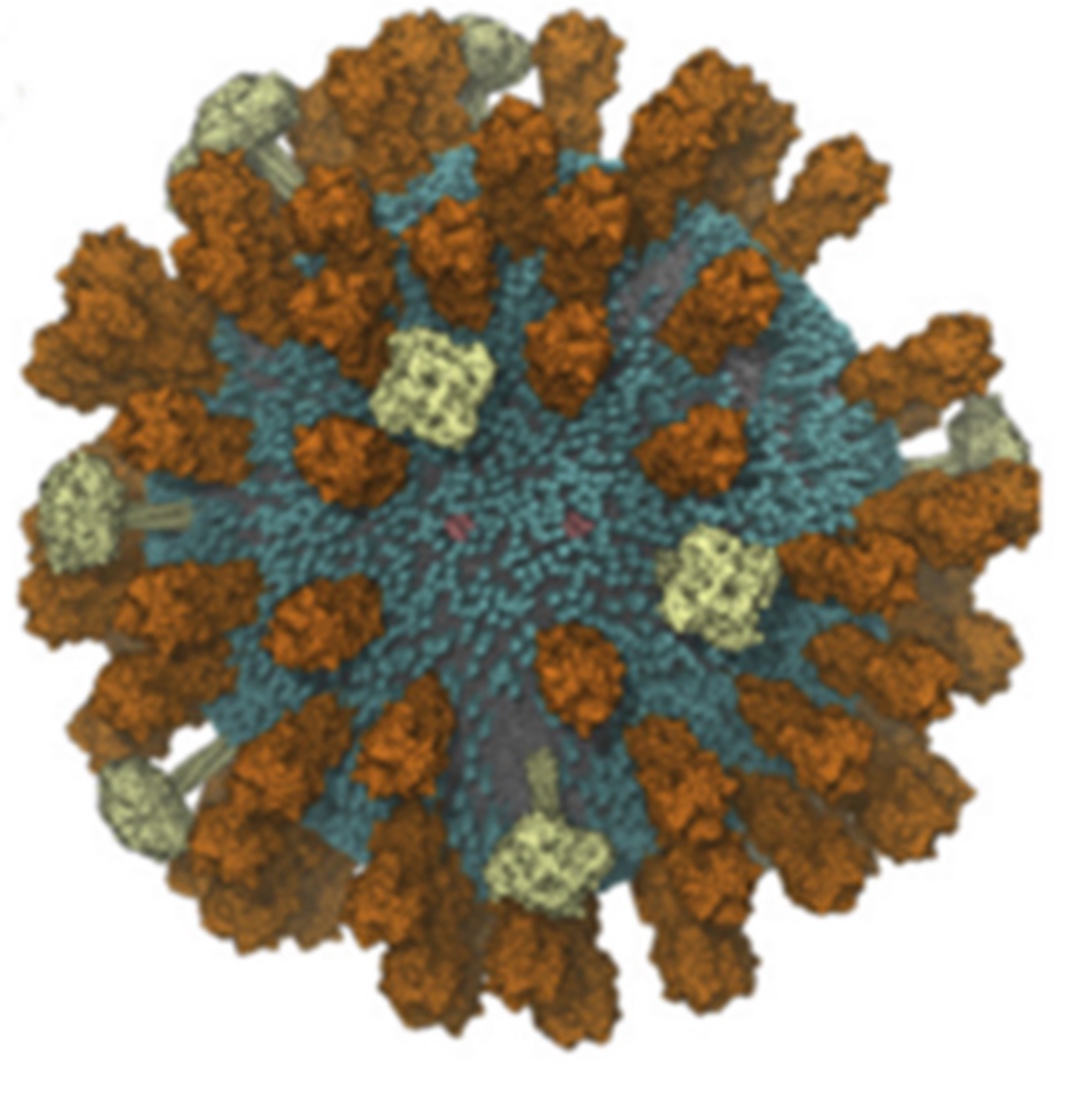 The need for detailed models and dynamics of virus particles is clear, as part of our fight against pandemics such as COVID. Given the size and composition of typical viruses, the use of Martini to simulate their behavior is apparent. Indeed, viruses have already been targeted with Martini, including the capsid of the Cowpea Mosaic virus [1], and enveloped ones such as Influenza A virion [2], Dengue [3], and flavivirus [4]. More examples are reviewed in [5,6].
The need for detailed models and dynamics of virus particles is clear, as part of our fight against pandemics such as COVID. Given the size and composition of typical viruses, the use of Martini to simulate their behavior is apparent. Indeed, viruses have already been targeted with Martini, including the capsid of the Cowpea Mosaic virus [1], and enveloped ones such as Influenza A virion [2], Dengue [3], and flavivirus [4]. More examples are reviewed in [5,6].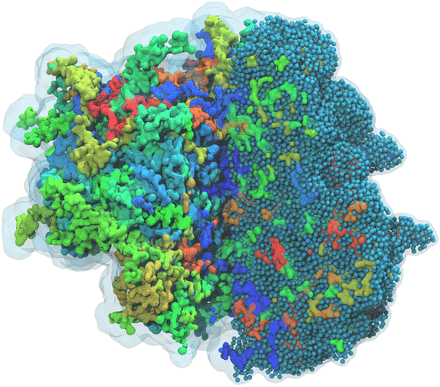 Intrinsically disorder proteins (IDPs) are an important class of proteins. Traditionally, the application of Martini to study IDPs has been limited due to the constraints in 2ndary structure imposed by an elastic network. However, the use of Go-potentials [1] as well as the use of SAXS constraints [2] offers more possibilities to capture the broad range of configurations characteristic of IDPs.
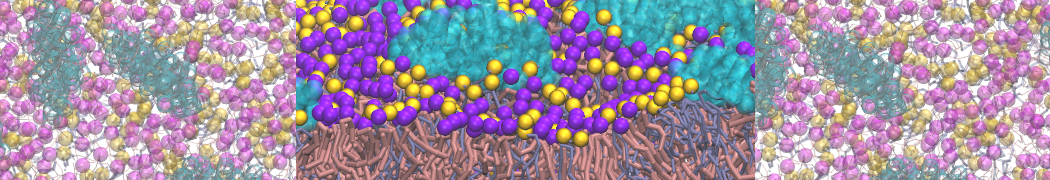
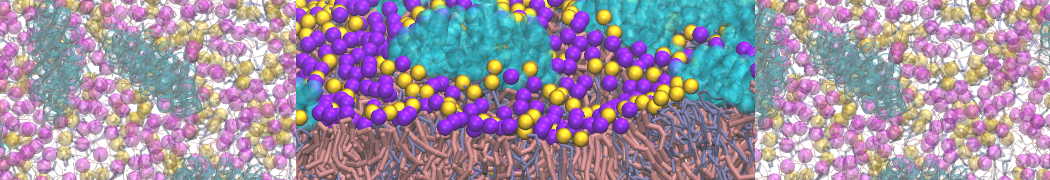
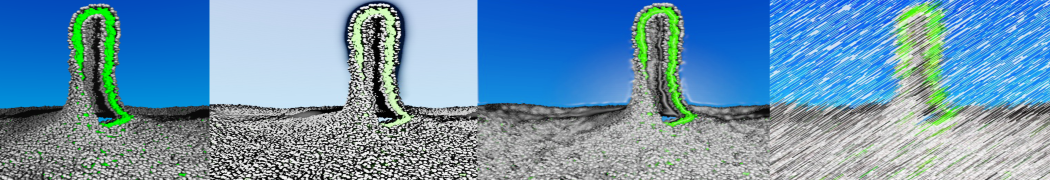
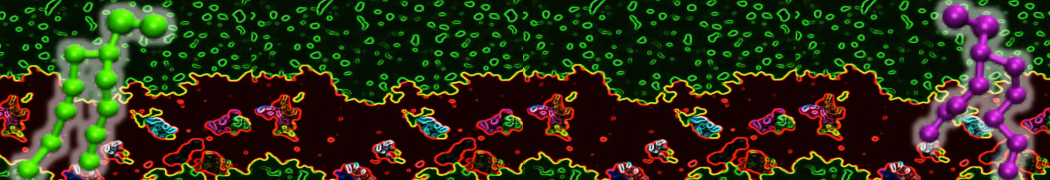
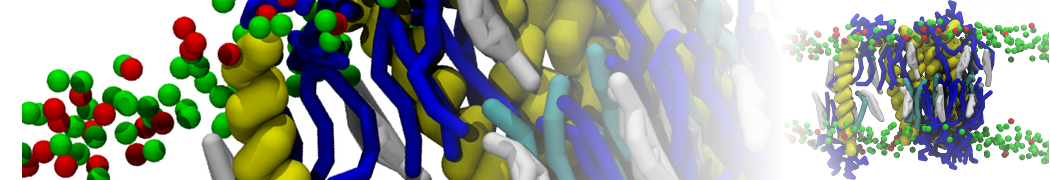
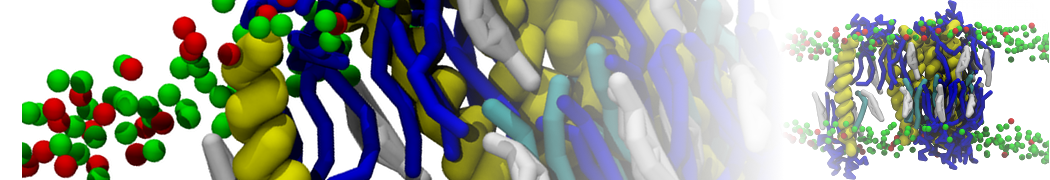
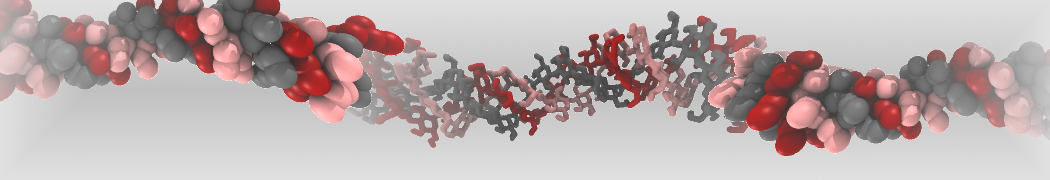
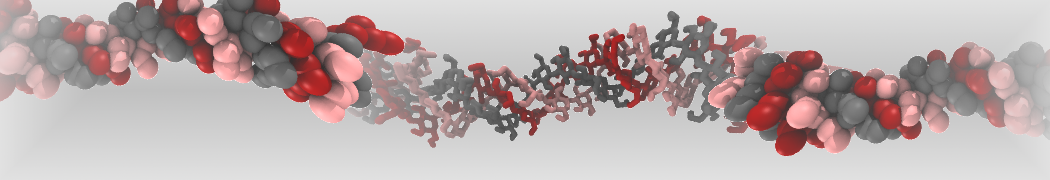
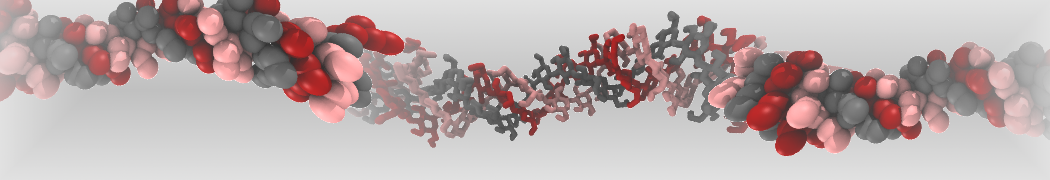
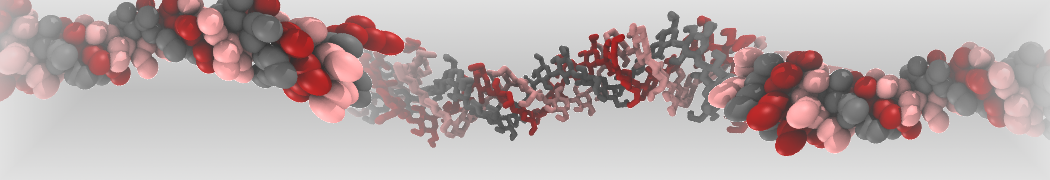
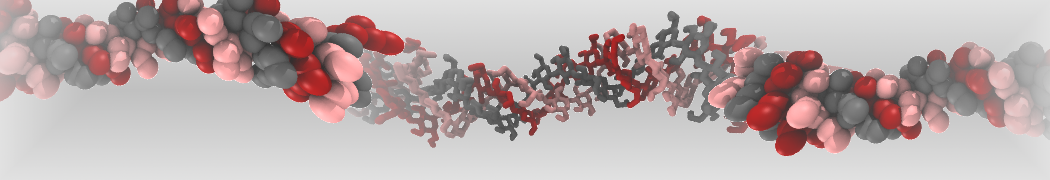
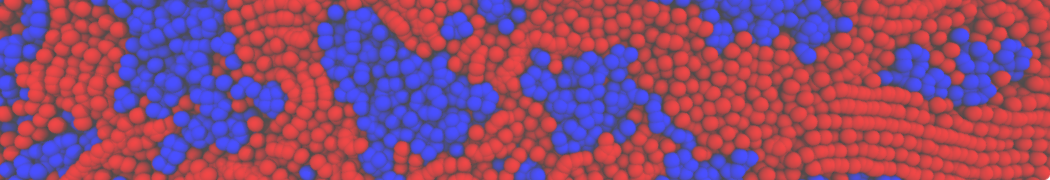
Intrinsically disorder proteins (IDPs) are an important class of proteins. Traditionally, the application of Martini to study IDPs has been limited due to the constraints in 2ndary structure imposed by an elastic network. However, the use of Go-potentials [1] as well as the use of SAXS constraints [2] offers more possibilities to capture the broad range of configurations characteristic of IDPs.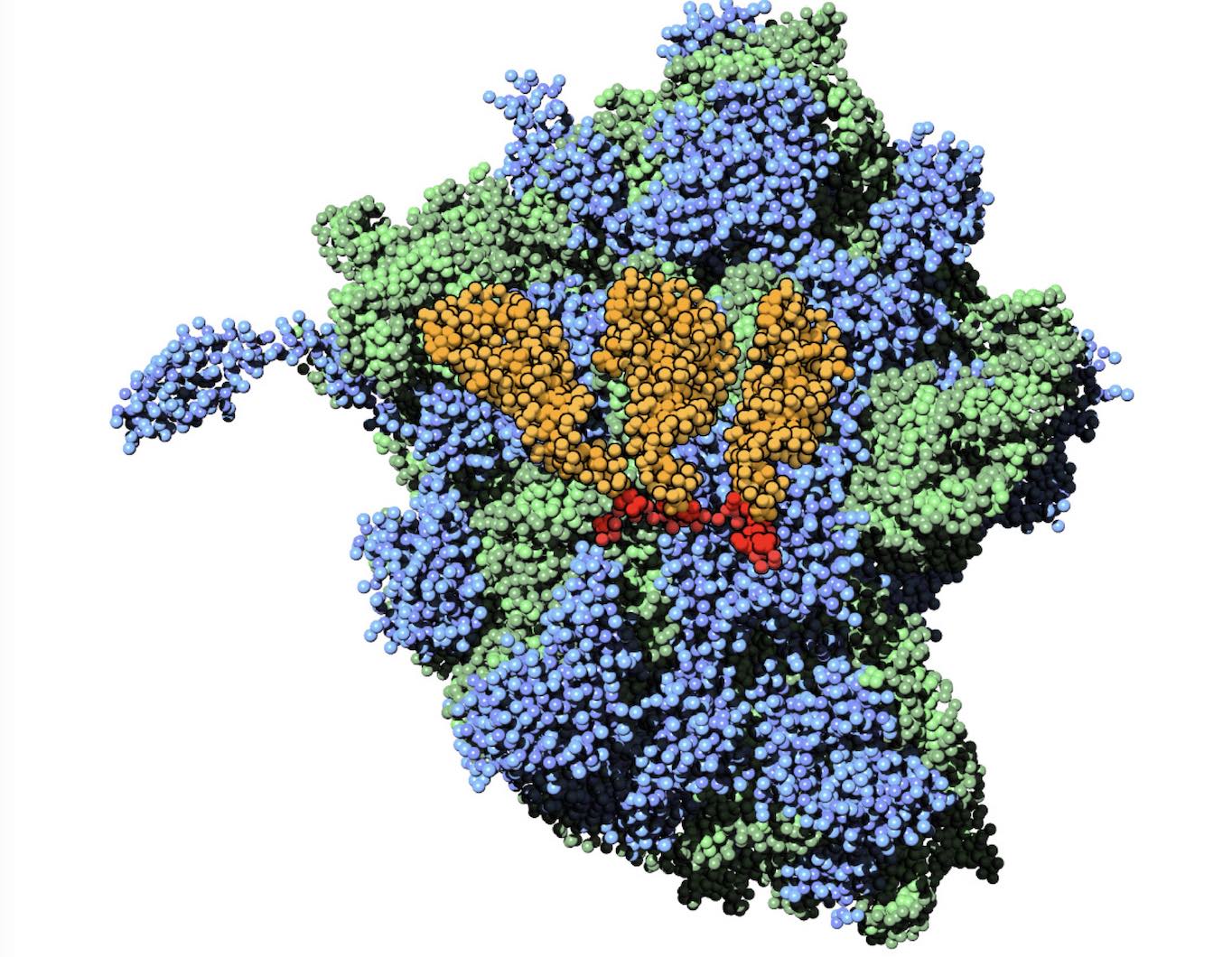 Polynucleotides (DNA, RNA) form a fundamental class of biomolecules. Given their importance, Martini models for both DNA [1] and RNA [2, see Figure depicting a Martini ribosome] have been developed. Parameters for nucleotide cofactors are also available [3,4], as well as a dedicated tool to analyze nucleoside packing [5]. Given the limitations of Martini with respect to modeling the directionality of hydrogen bonds, nucleotide folding and hybridization events can not be captured. As for the protein model, an elastic network is required to keep the overall structure in place.
Polynucleotides (DNA, RNA) form a fundamental class of biomolecules. Given their importance, Martini models for both DNA [1] and RNA [2, see Figure depicting a Martini ribosome] have been developed. Parameters for nucleotide cofactors are also available [3,4], as well as a dedicated tool to analyze nucleoside packing [5]. Given the limitations of Martini with respect to modeling the directionality of hydrogen bonds, nucleotide folding and hybridization events can not be captured. As for the protein model, an elastic network is required to keep the overall structure in place. Biomembranes are essential cellular components. Together with membrane-adhered structures, such as the cytoskeleton, cell membranes constitute incredibly heterogeneous and crowded environments, containing hundreds of different lipid types and being densely packed with a large variety of membrane proteins. They provide identity not only to the cell as a whole, through the enveloping plasma membrane, but also to many internal organelles.
Biomembranes are essential cellular components. Together with membrane-adhered structures, such as the cytoskeleton, cell membranes constitute incredibly heterogeneous and crowded environments, containing hundreds of different lipid types and being densely packed with a large variety of membrane proteins. They provide identity not only to the cell as a whole, through the enveloping plasma membrane, but also to many internal organelles.