Martini Artefacts
- Details
- Last Updated: Tuesday, 23 March 2021 15:43



 General Purpose Coarse-Grained Force Field
General Purpose Coarse-Grained Force Field 
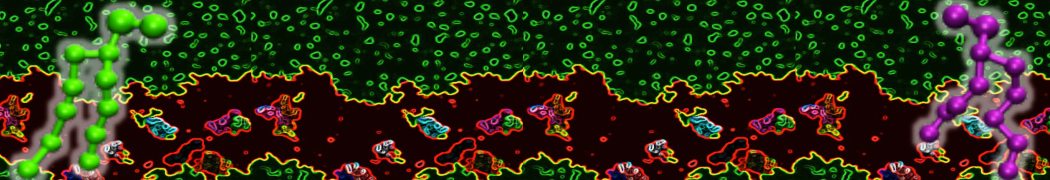
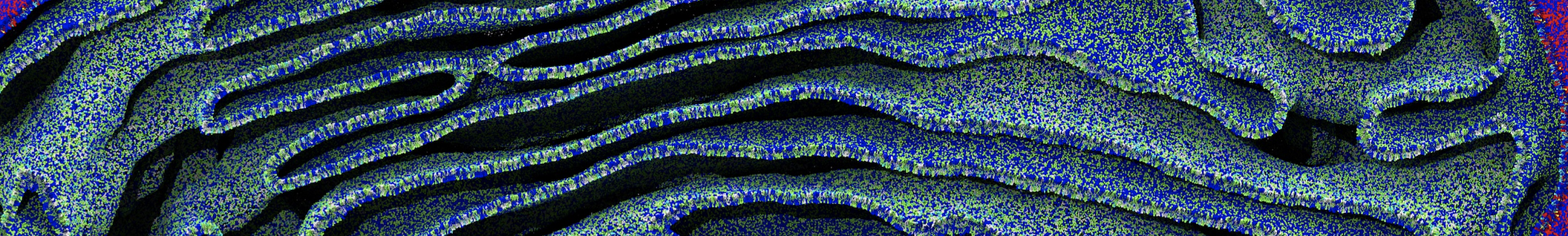
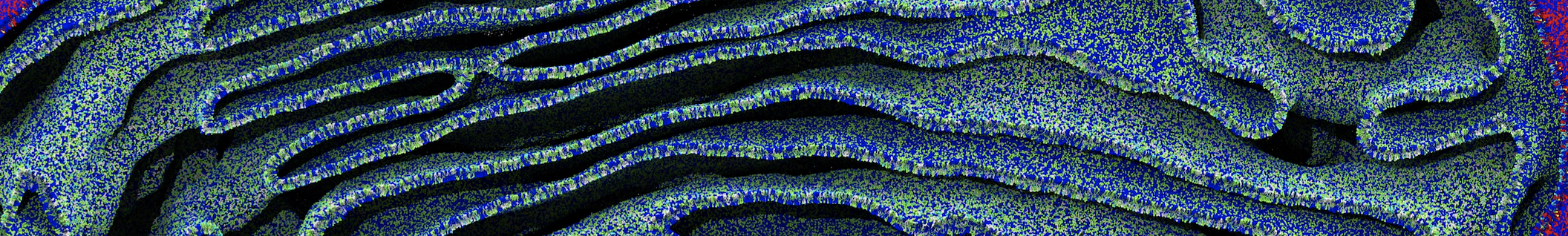
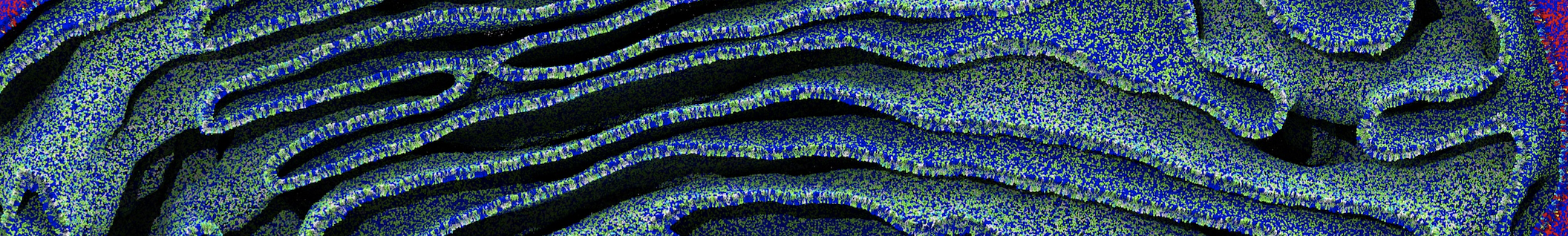
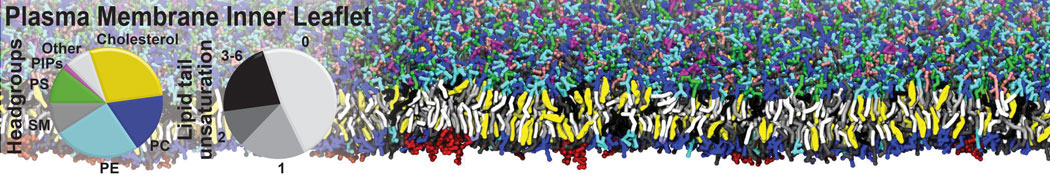
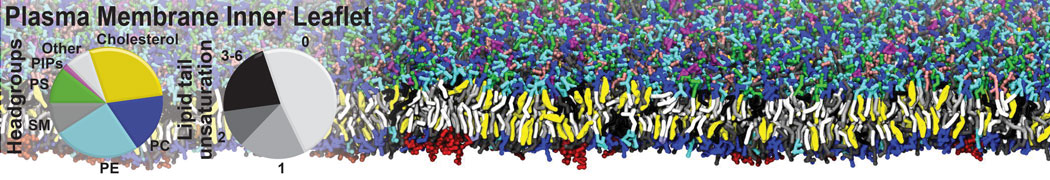
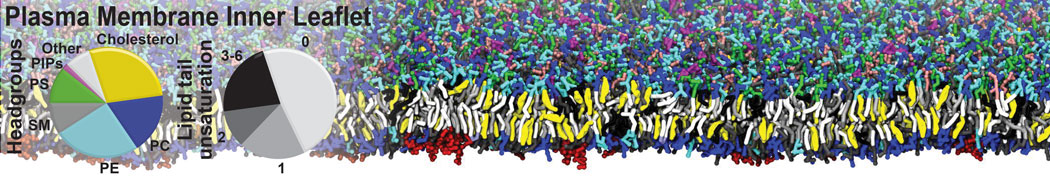
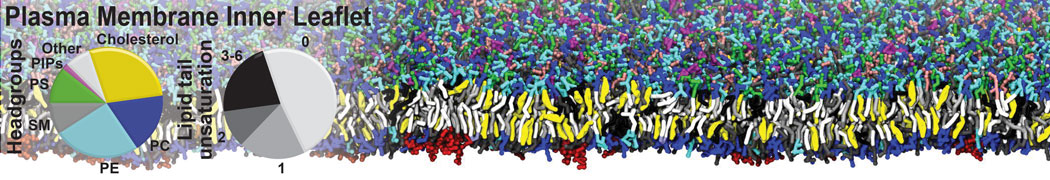
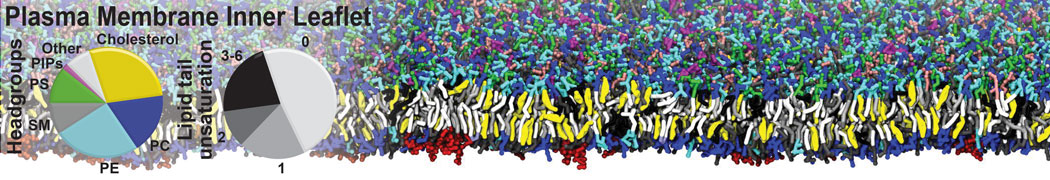
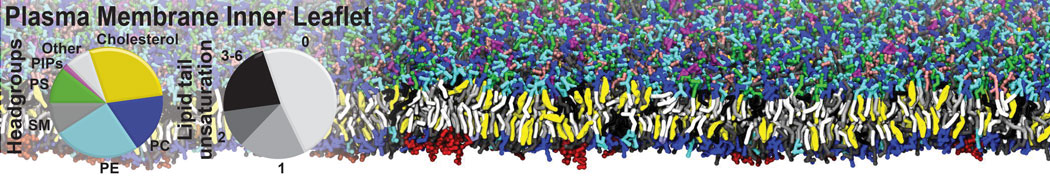
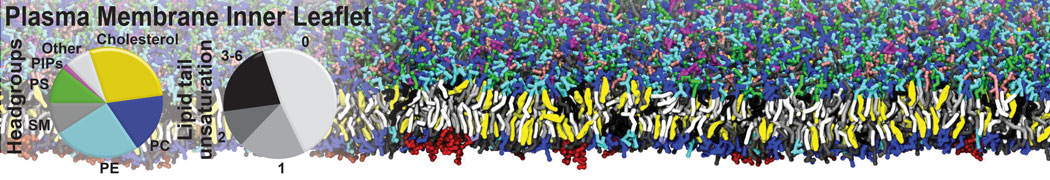
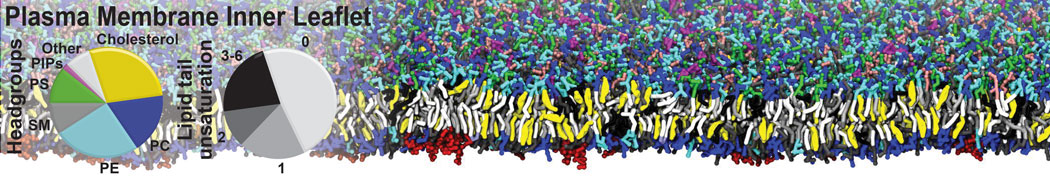


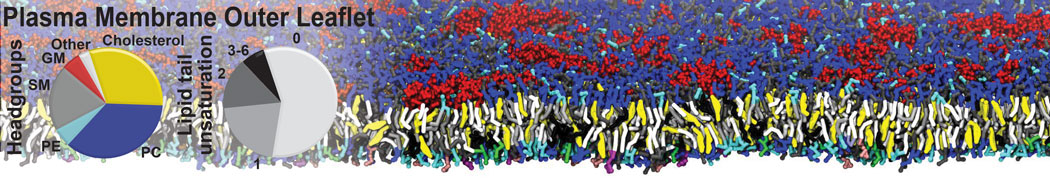
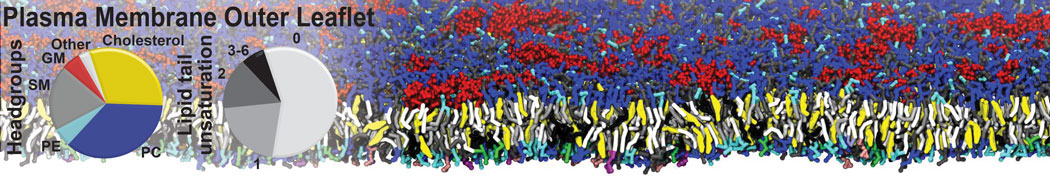
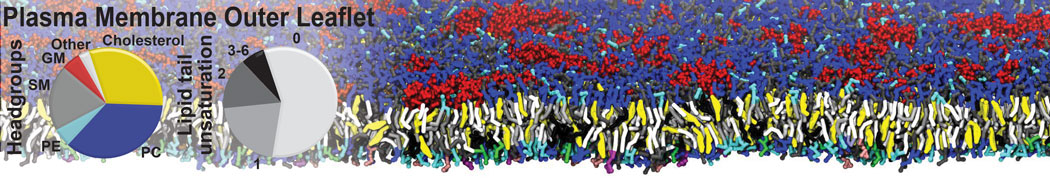
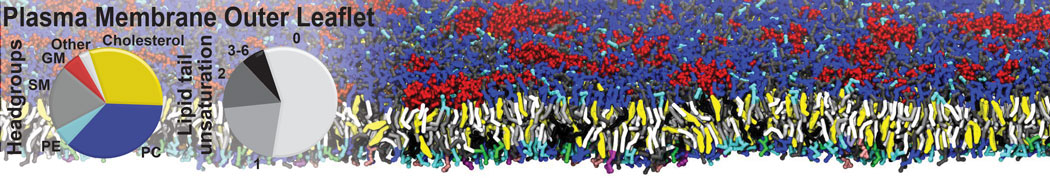
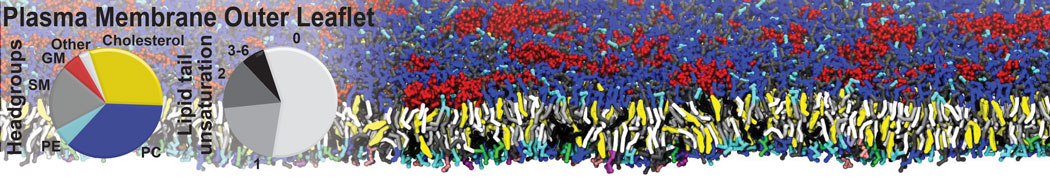
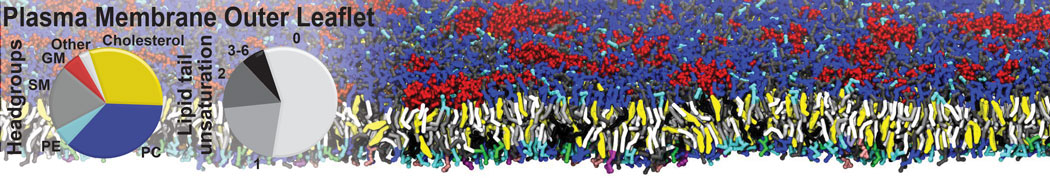
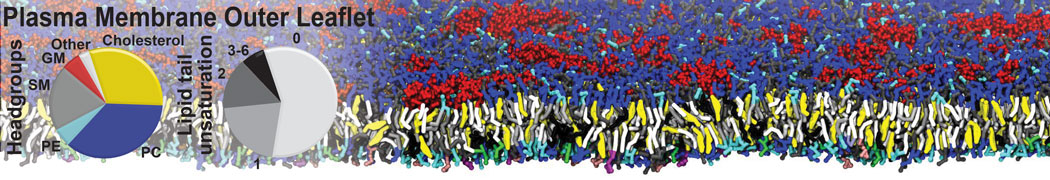
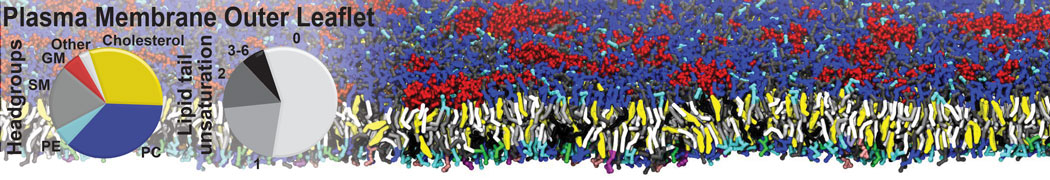
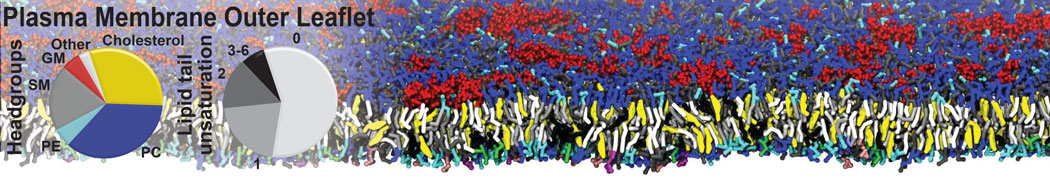
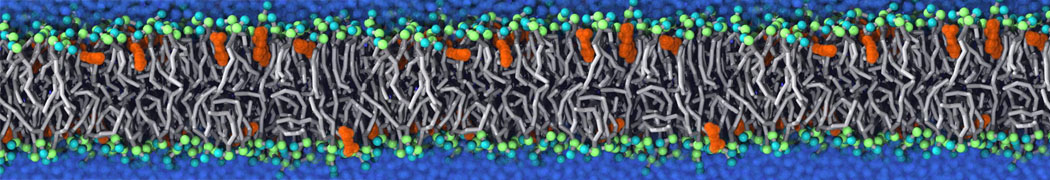
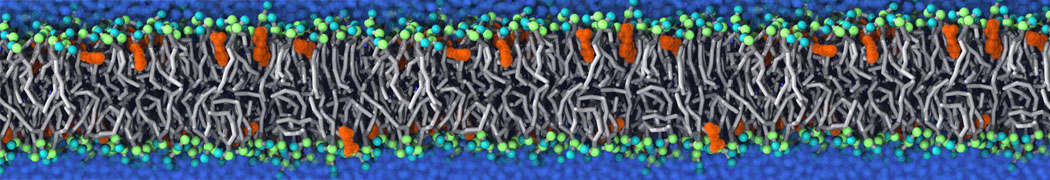
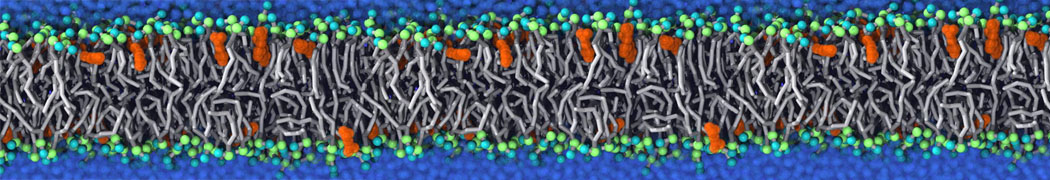
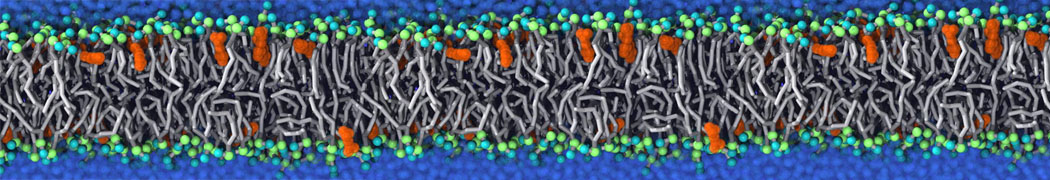
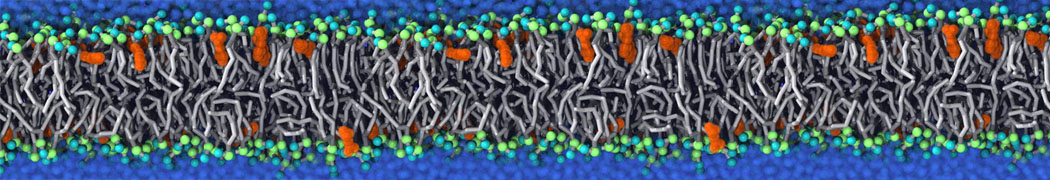
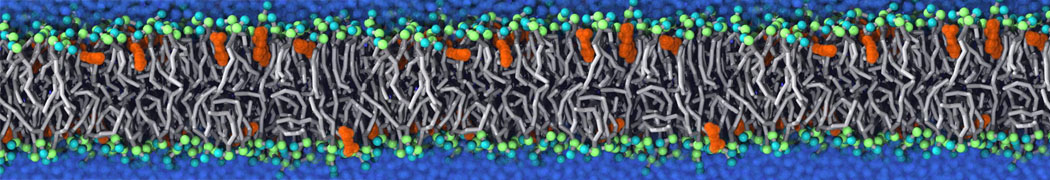
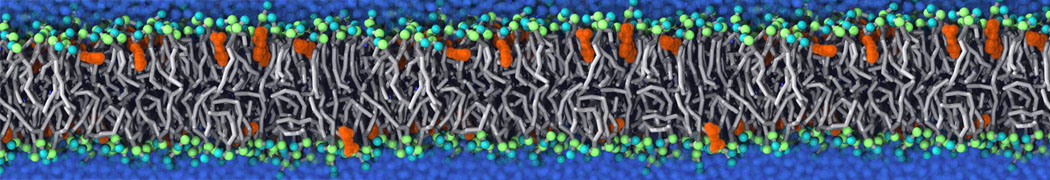
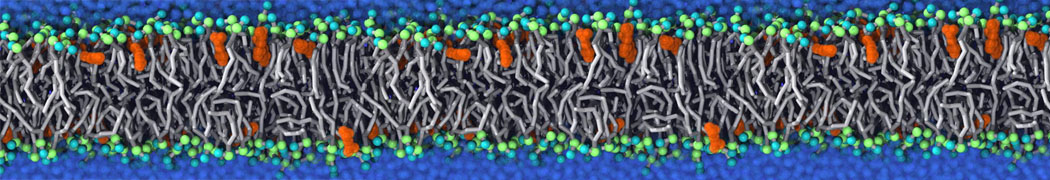
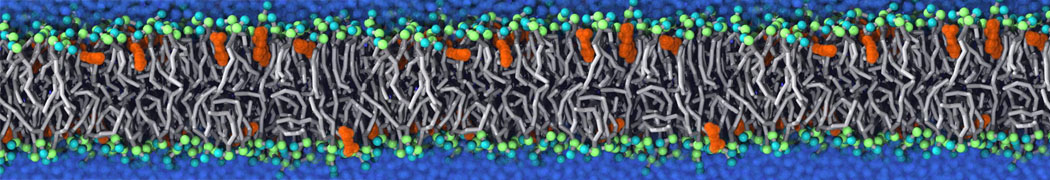
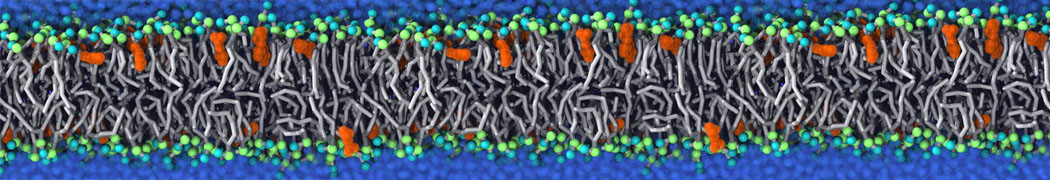





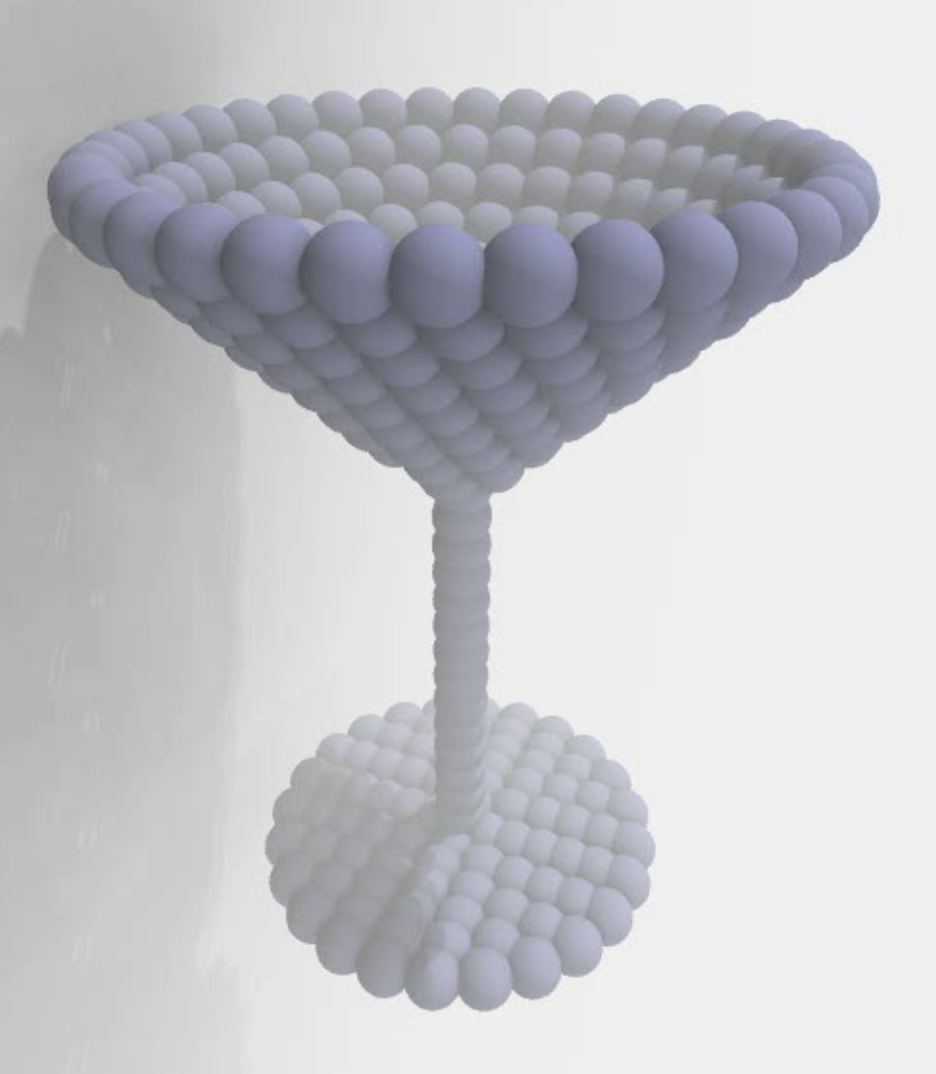
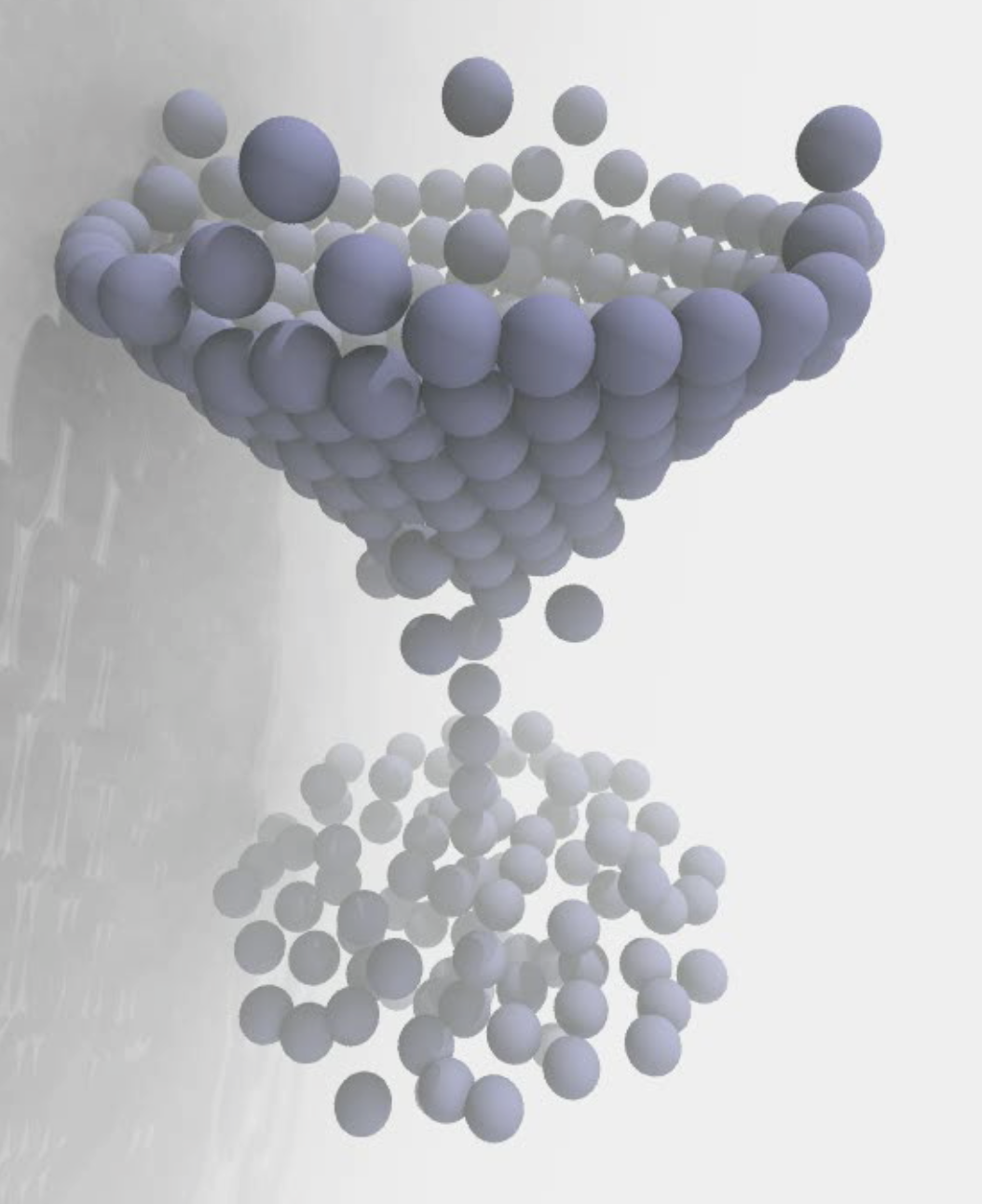
Membranes provide identity not only to the cell as a whole, through the enveloping plasma membrane, but also to many internal organelles. Membrane spatial organization ranges from a locally flat, lamellar geometry to highly curved ones as can be found, for instance, in the endoplasmic reticulum, Golgi apparatus, thylakoids, or mitochondria. Moreover, these structures are dynamic, involved in many remodelling processes often with highly curved intermediates.
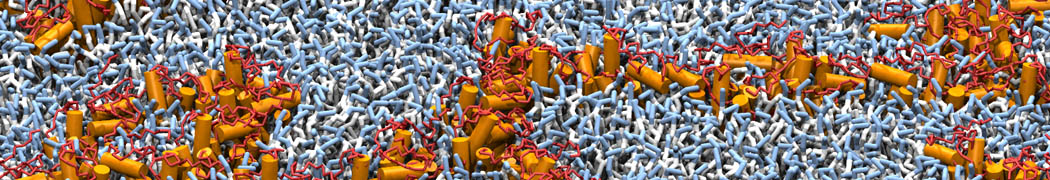
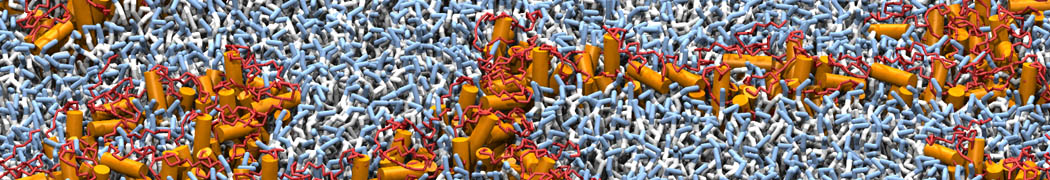
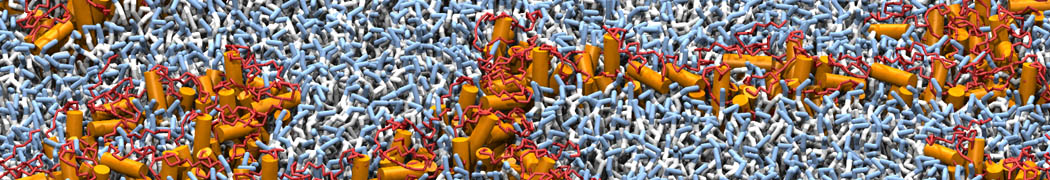
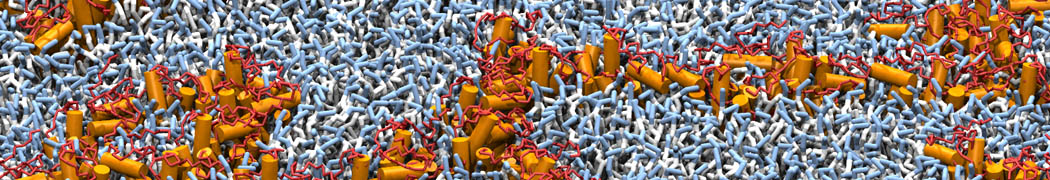
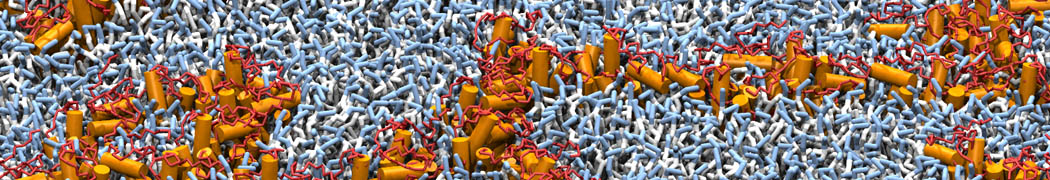
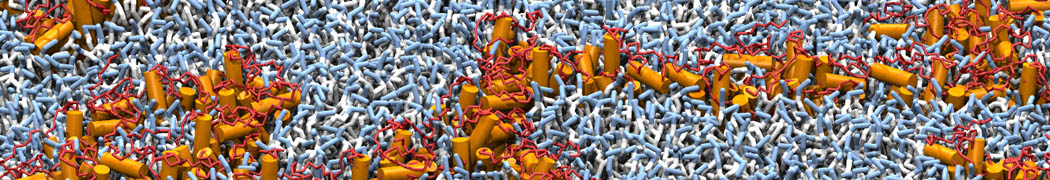
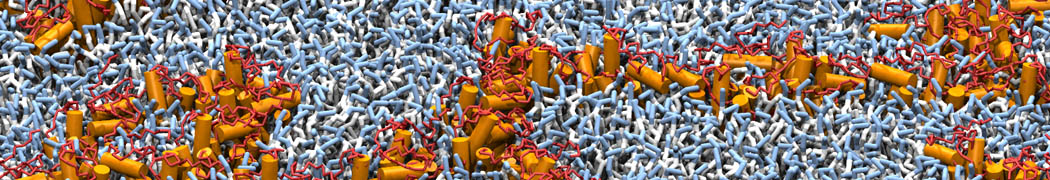
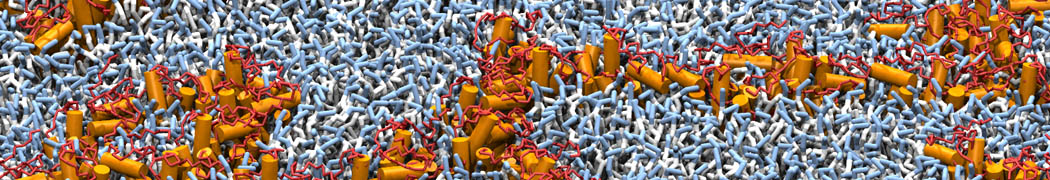
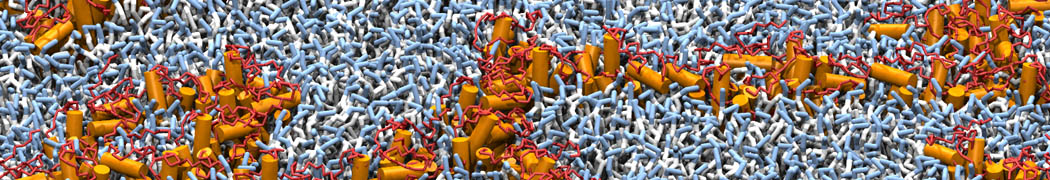
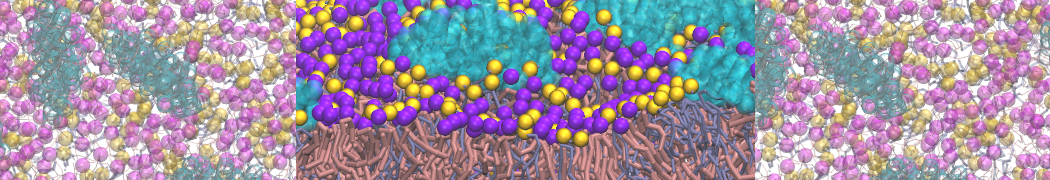
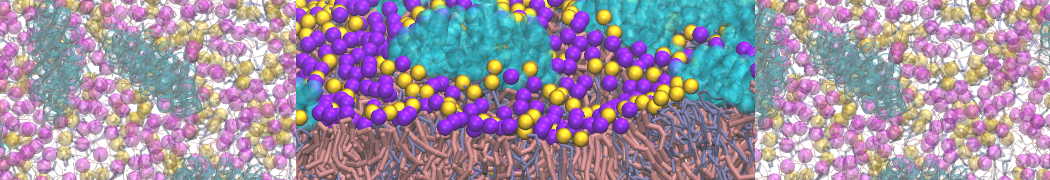
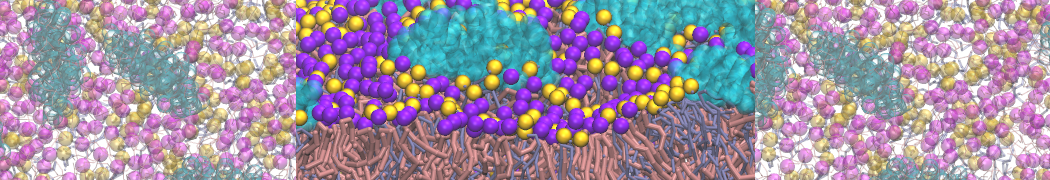
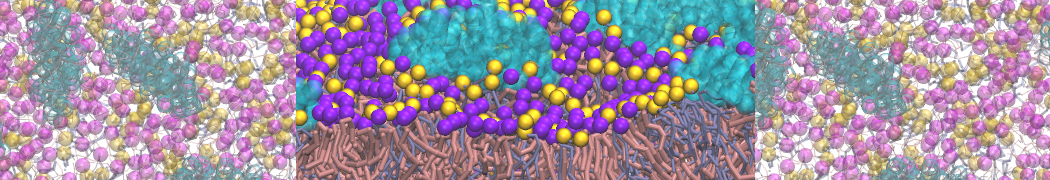
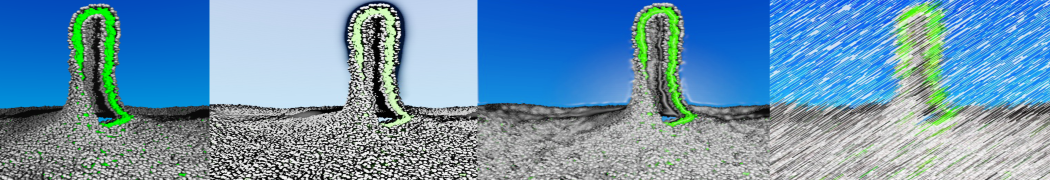
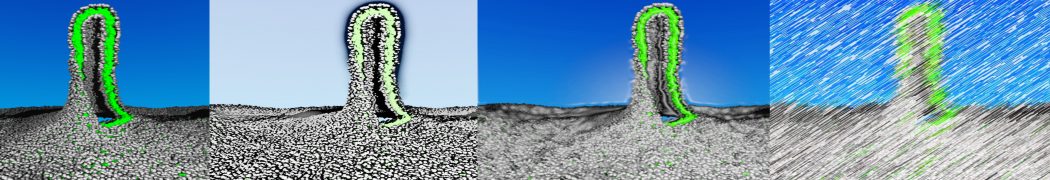
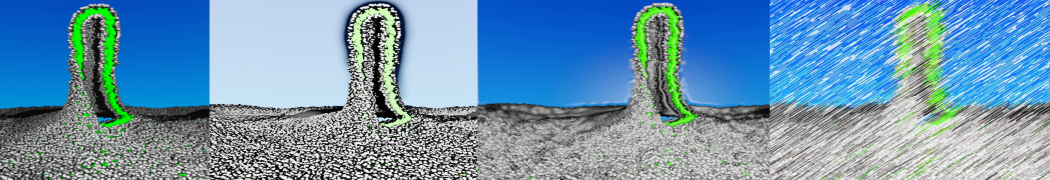
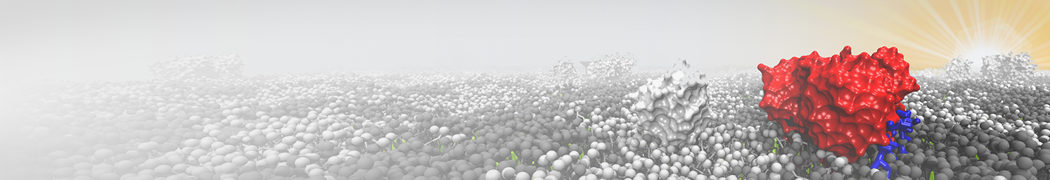
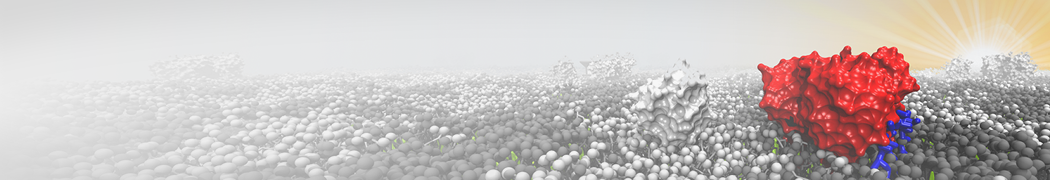
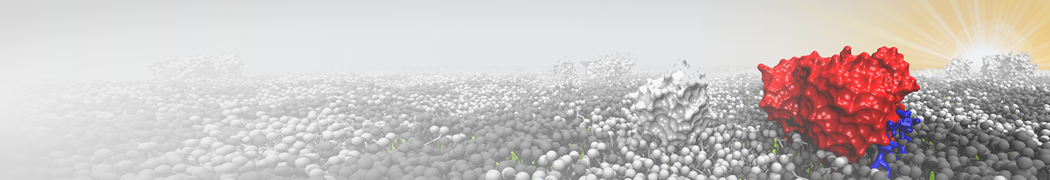
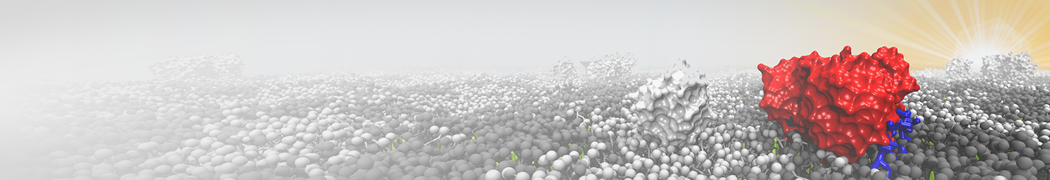
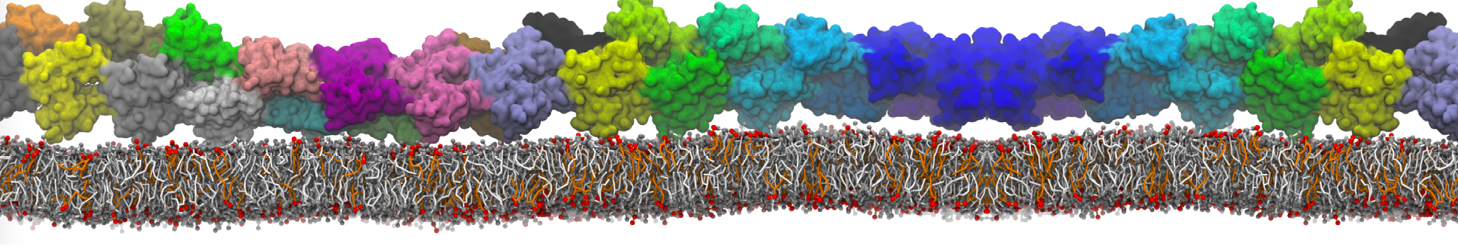
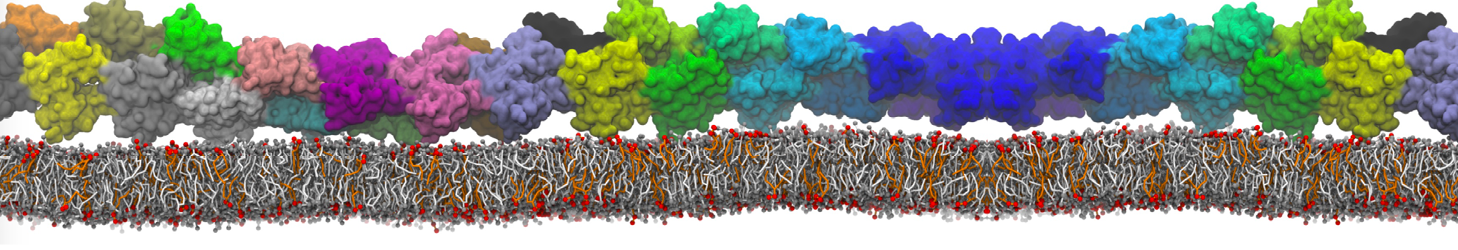
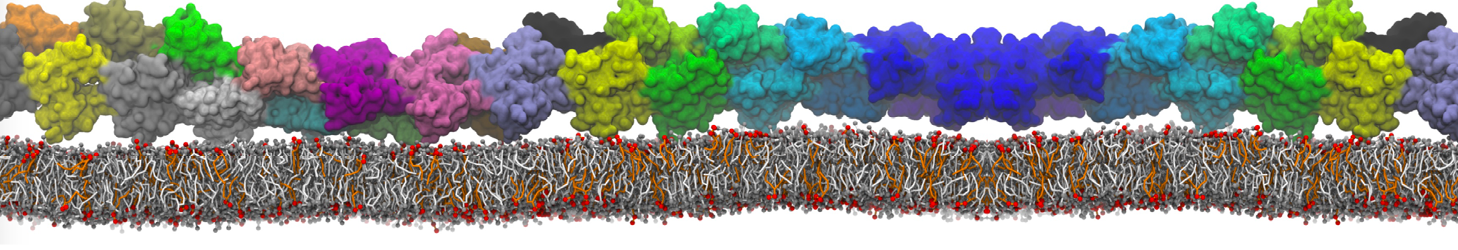
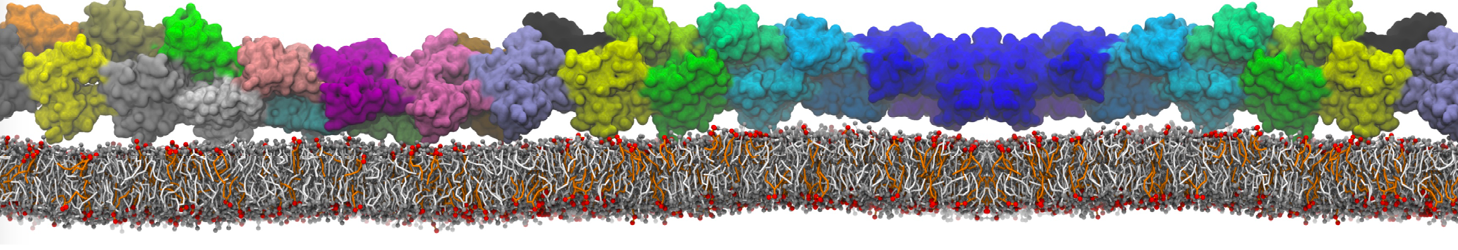
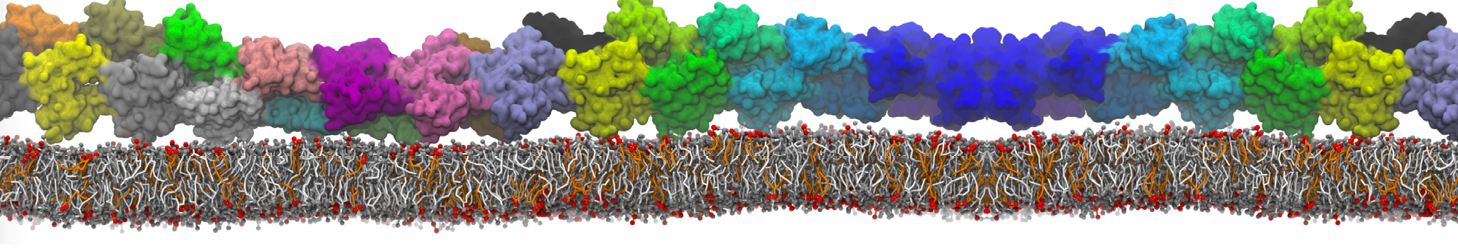
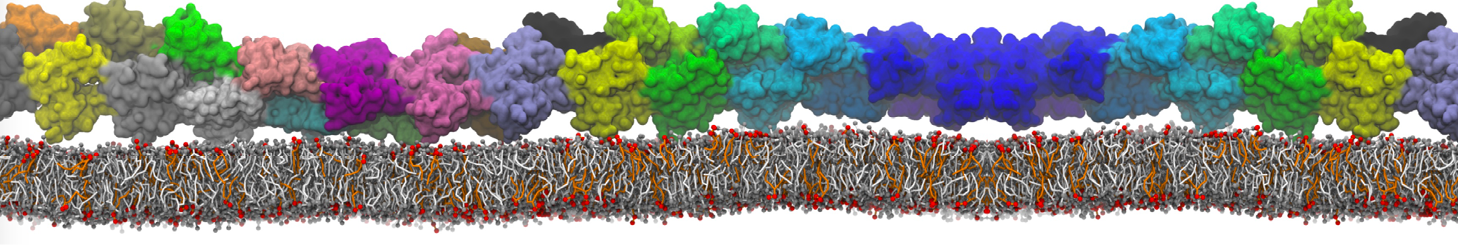
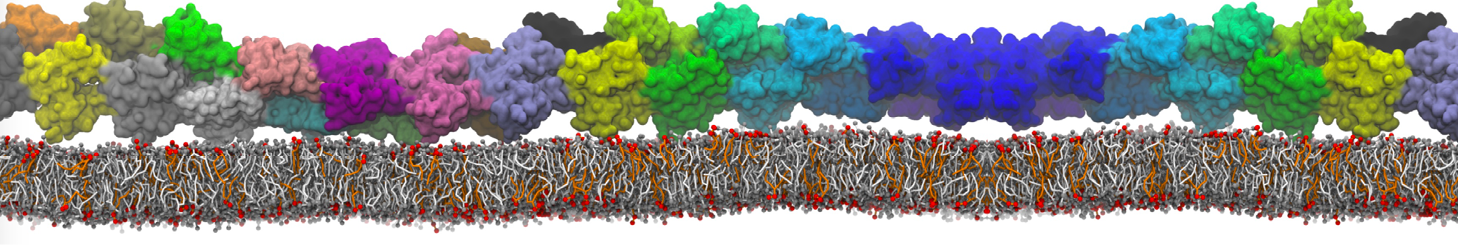
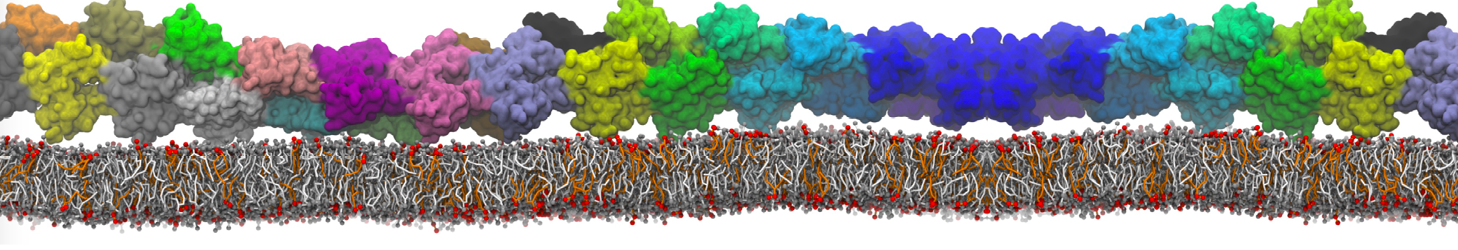
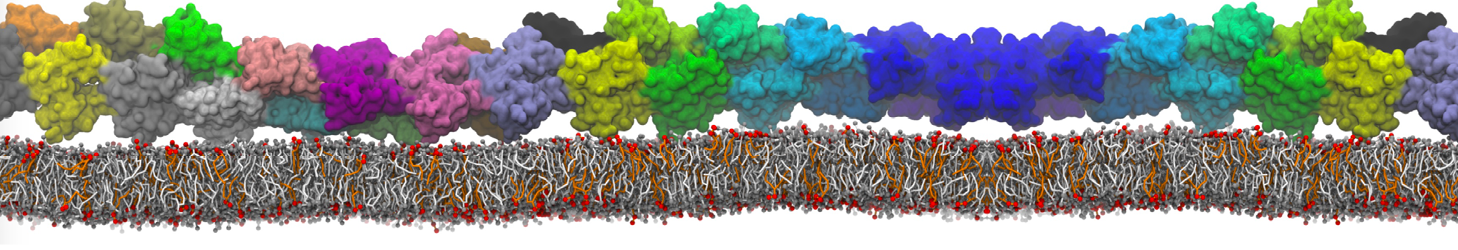
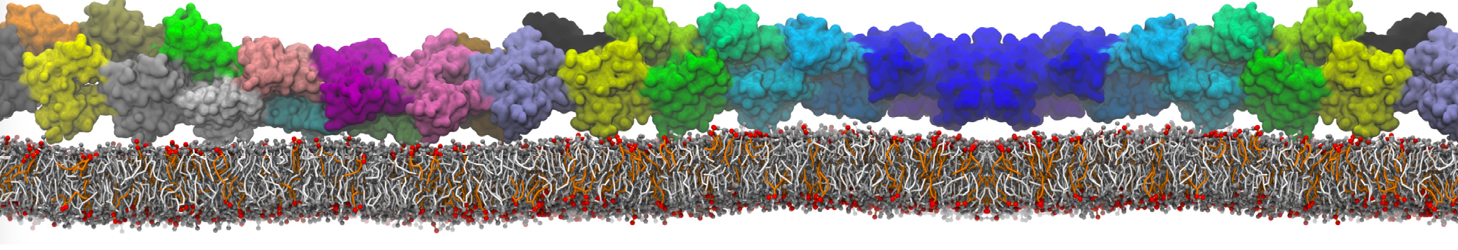
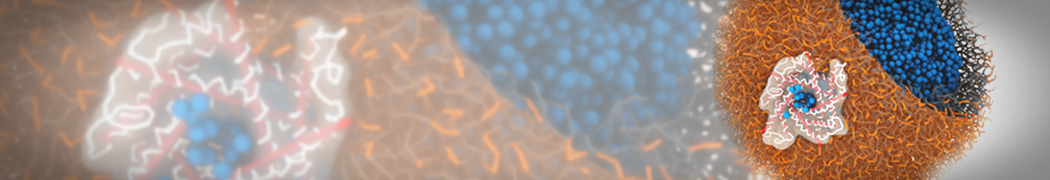
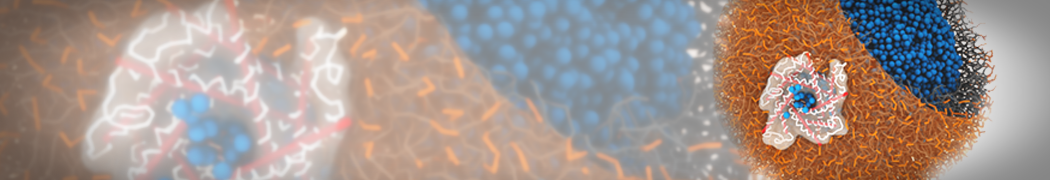
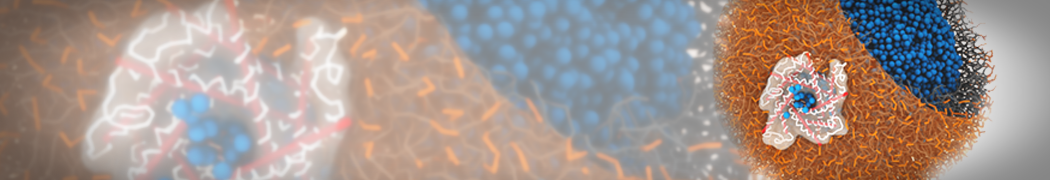
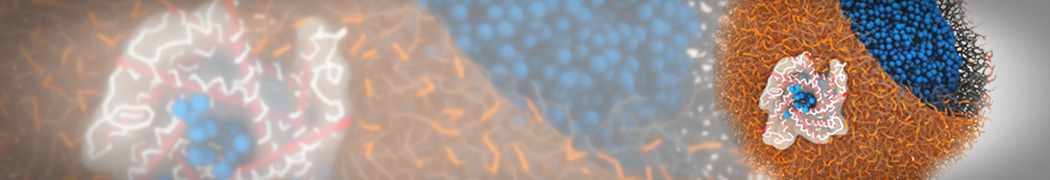
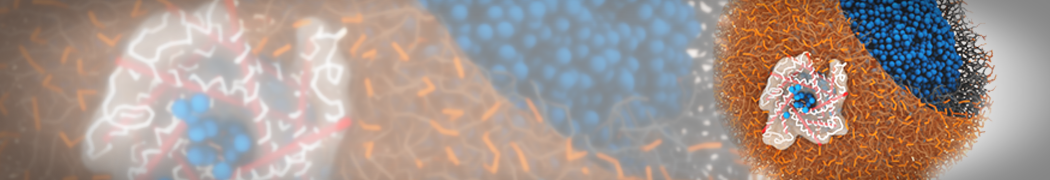
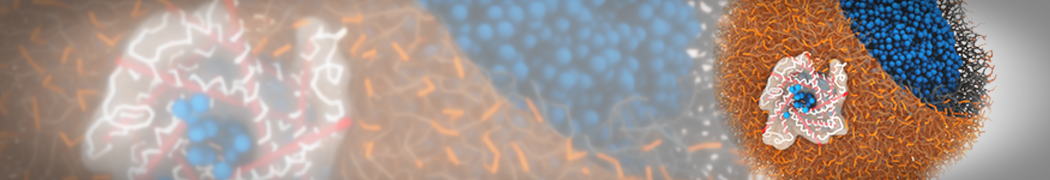
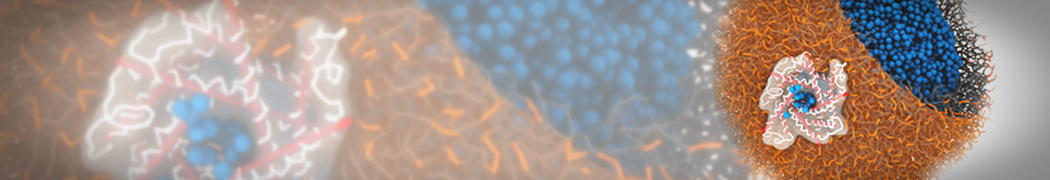
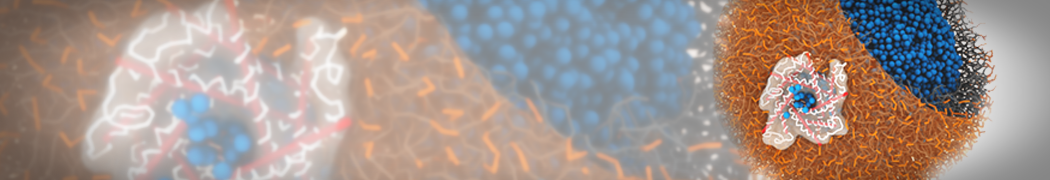
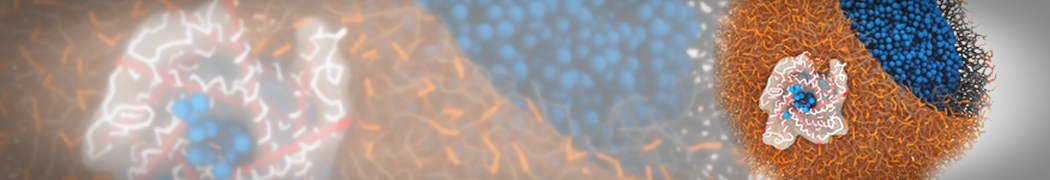
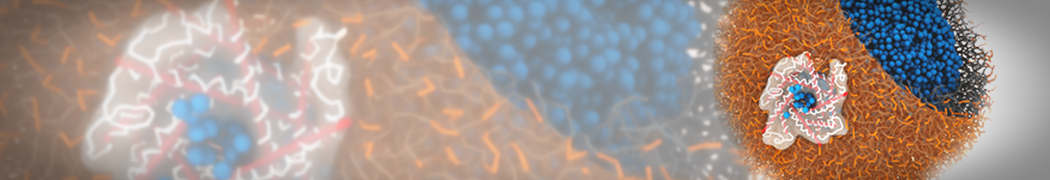
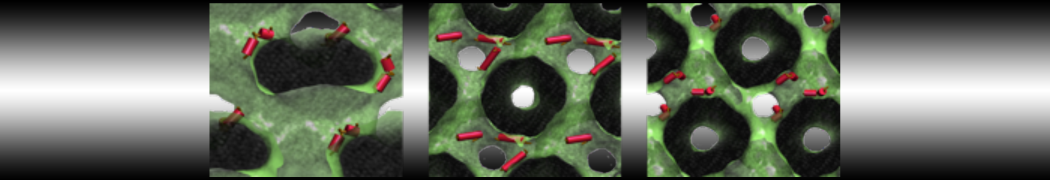
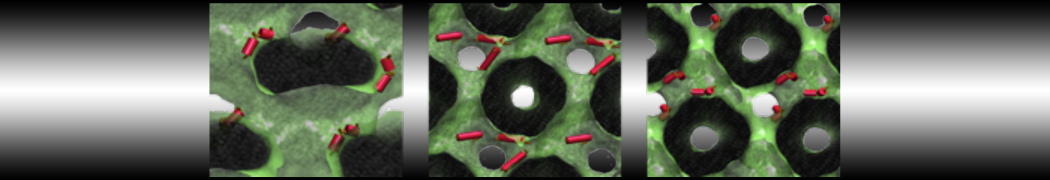
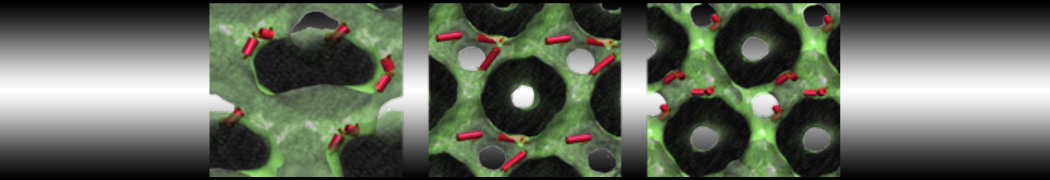
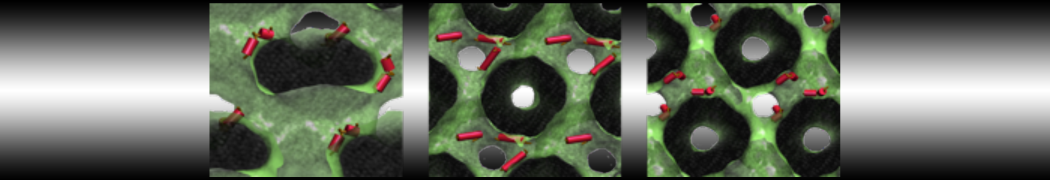
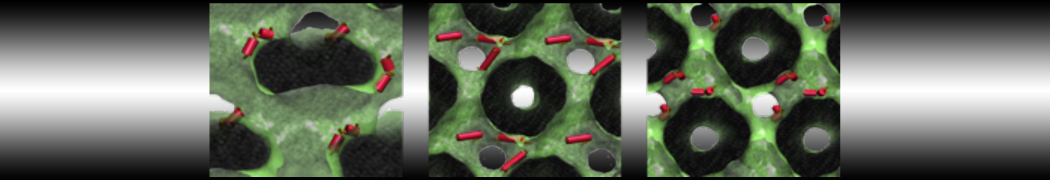
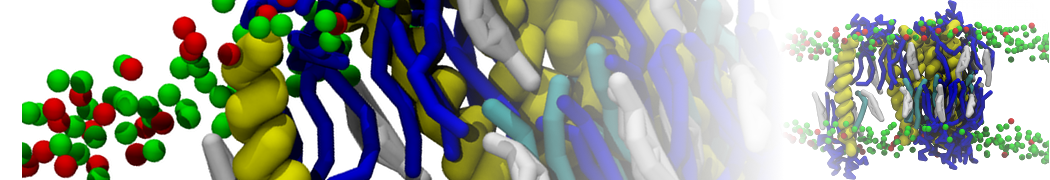
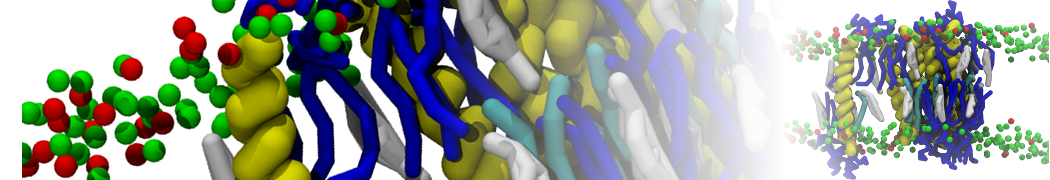
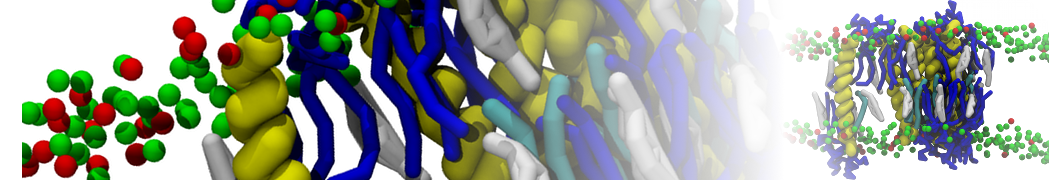
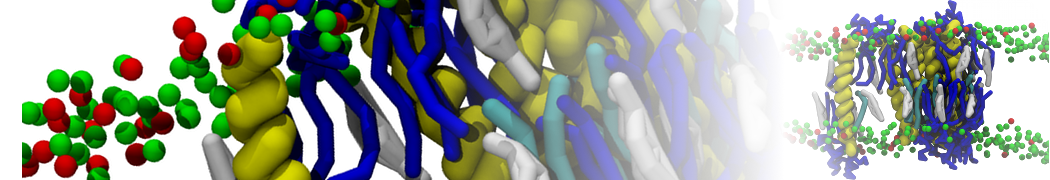
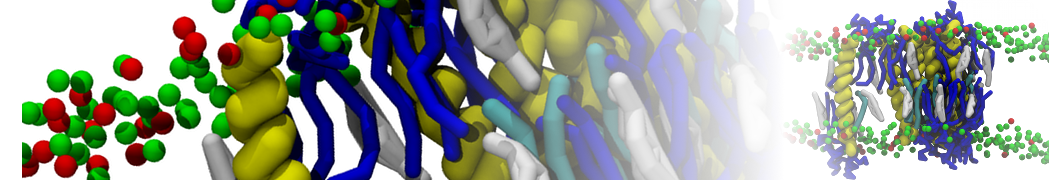
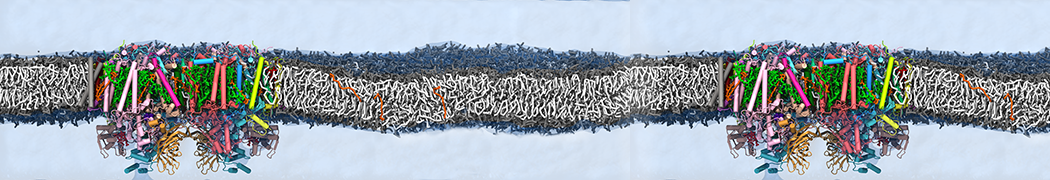
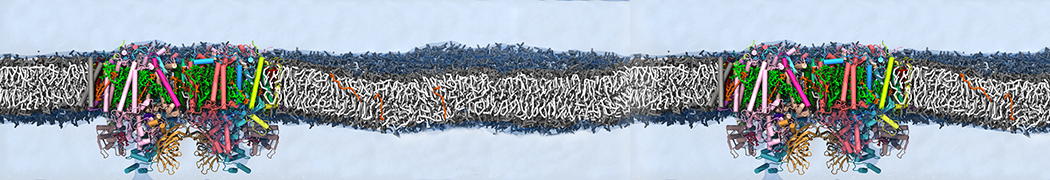
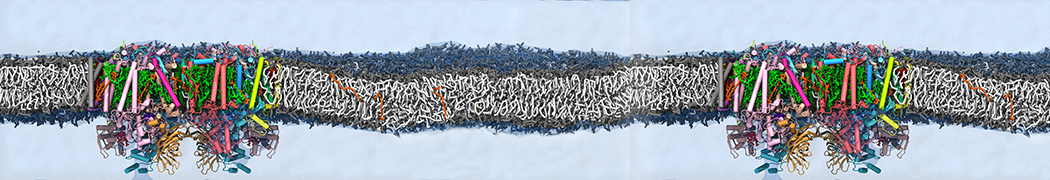
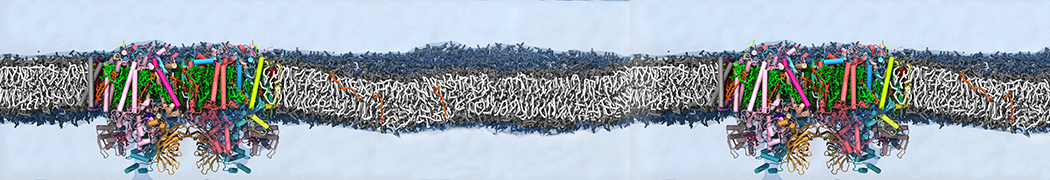
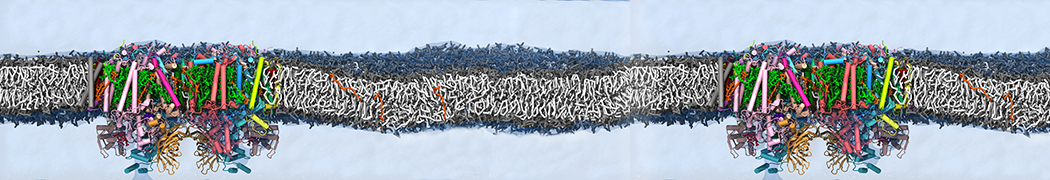
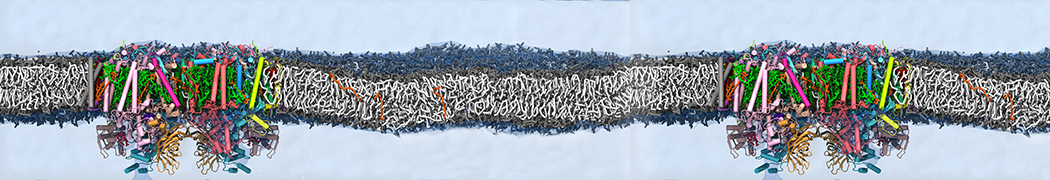
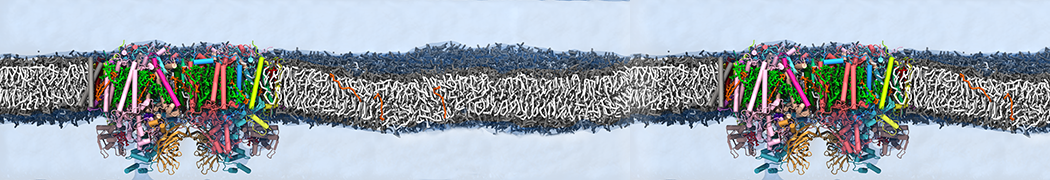
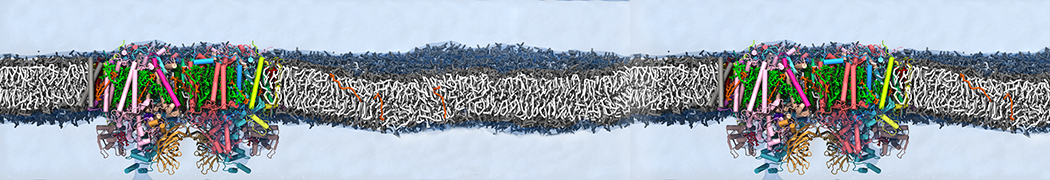
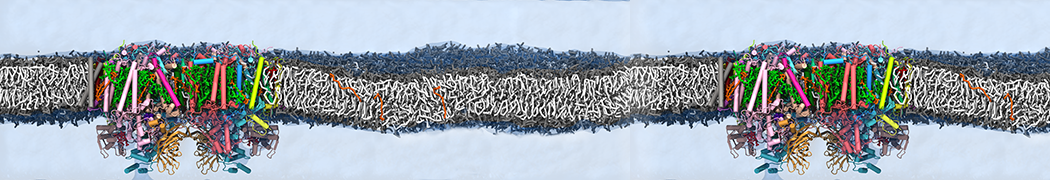
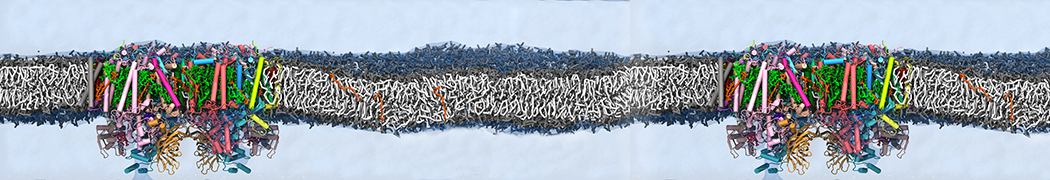
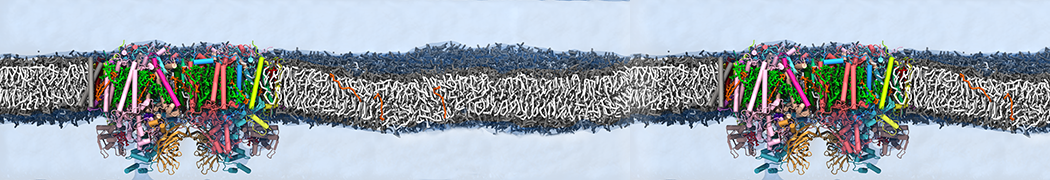
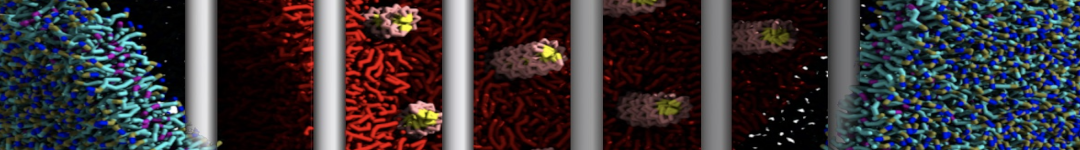
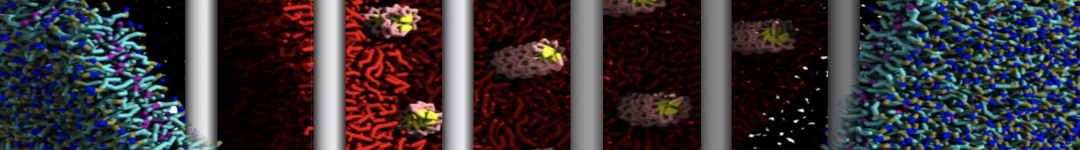
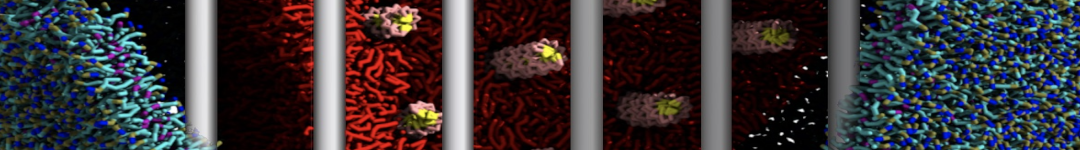
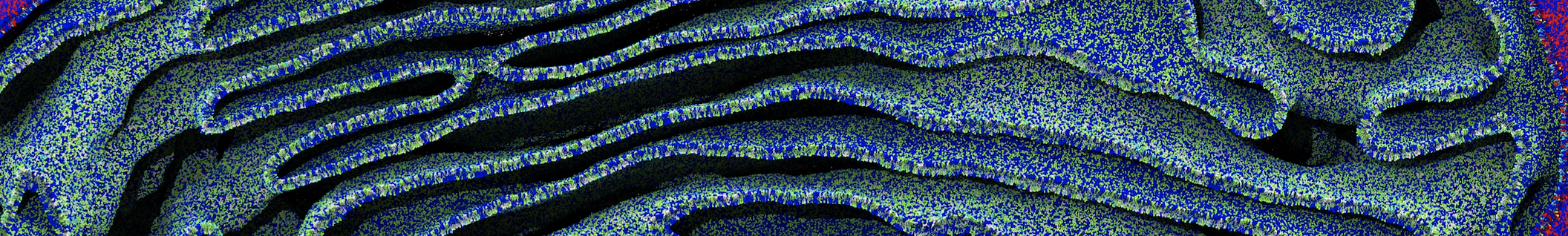
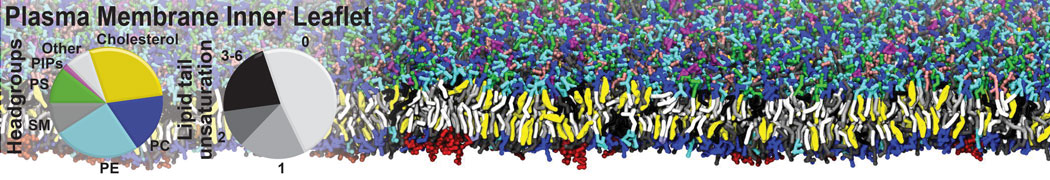
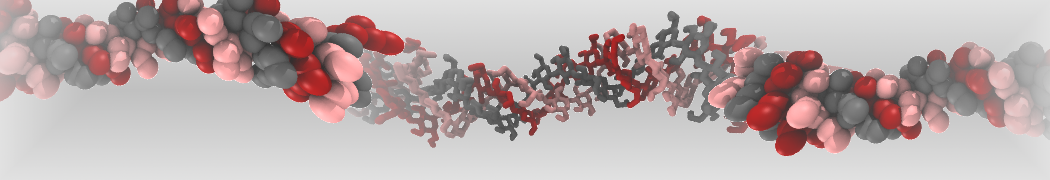
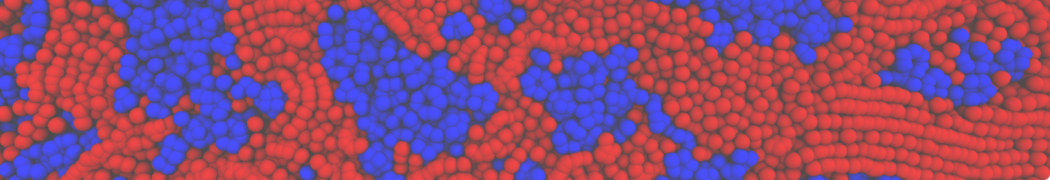
Martini-based simulations have proven useful in capturing these membrane shapes, from the strongly curved regions present in small lipid vesicles [1,2] and lipid tethers [3], to the large scale undulations of planar membrane patches. These type of simulations provide important clues about the interplay between curvature and protein/lipid sorting (see Figure, taken from [4]).
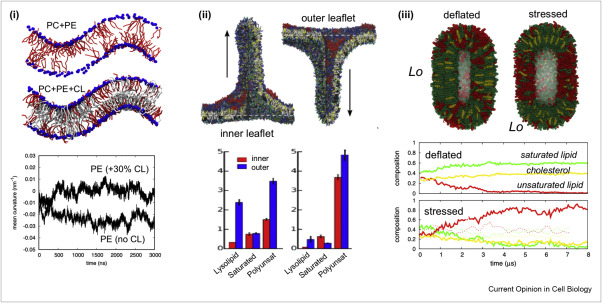
Recently, efforts are directed in modeling realistic shapes of large cellular substructures and even entire organelles, such as the mitochondrion [5]. A sophisticated tool to generate Martini membranes with arbitrary curvatures is TS2CG [5,6], and a tool to identify lipid leaflets in complex curved membranes is also available [7].
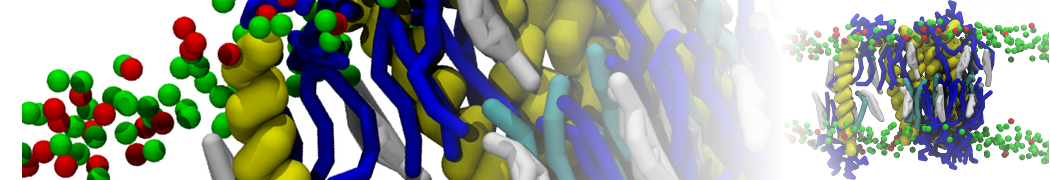
In addition, recent Martini-based simulation efforts are directed to understand membrane curvature generation via protein-lipid interplay. Recent examples include the strong membrane deformations induced by ECF transporters [8] and around a signal peptidase [9], and membrane deformation induced by functionalized oligonucleotides [10].
Using top-secret parameters, Martini now also simulates full-body humans:
Movies of the girl and boy in a simulated tap-dancing routine can be downloaded: (girl, boy)
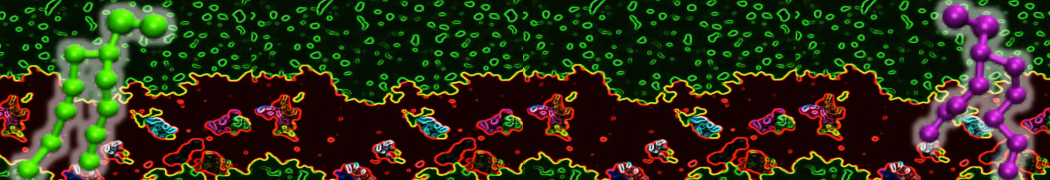
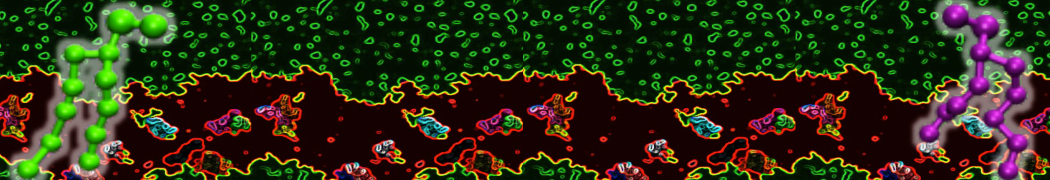
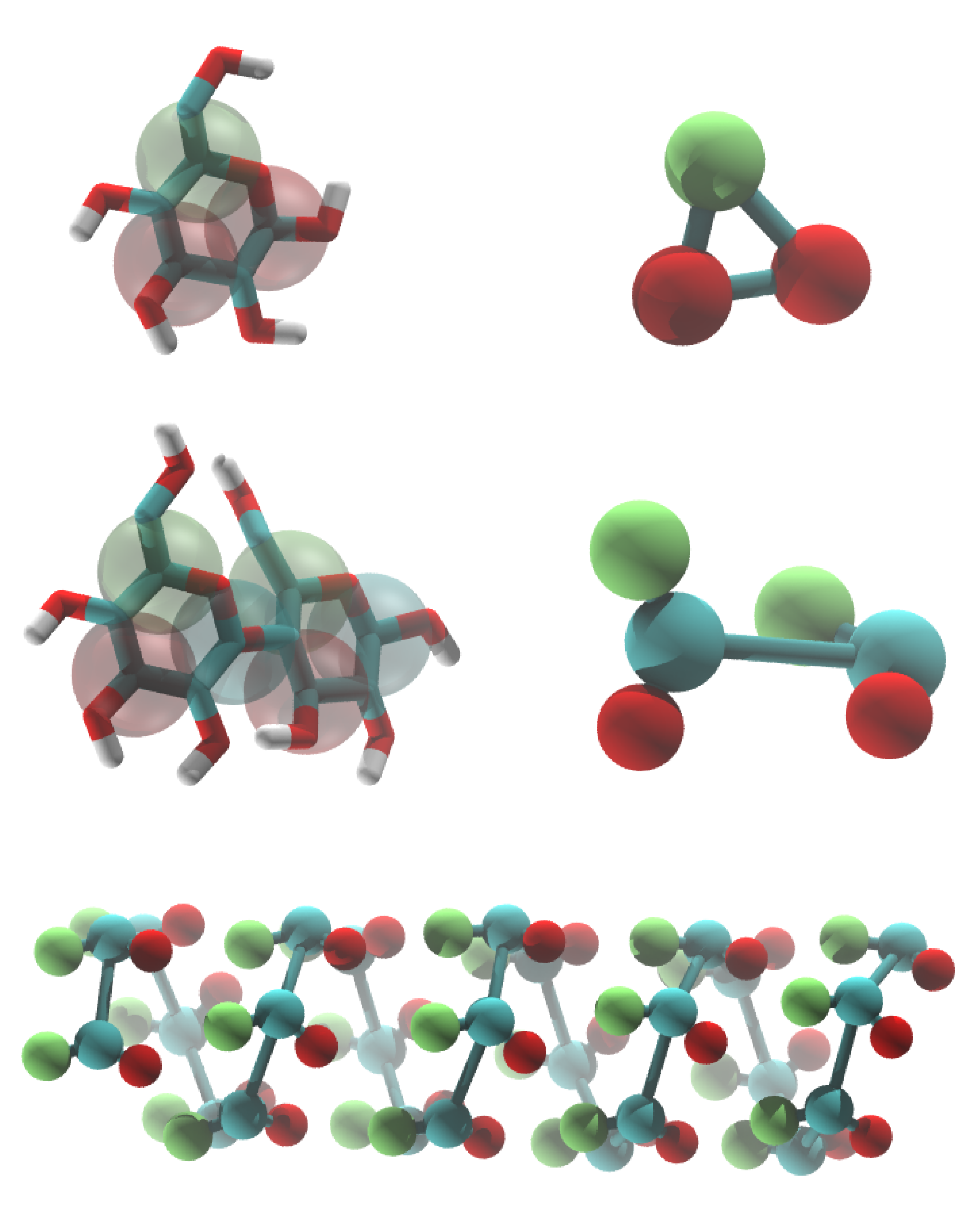 Carbohydrates (saccharides), the most abundant product of photosynthesis, play an important role in the energetic metabolism of living species and the signaling and immunological responses and are a fundamental component of the external cell wall of many organisms. In addition, saccharides are present in a variety of emerging classes of biomimetic materials. Furthermore, due to their cryo- and anhydro-protective properties, many sugars have been shown to be effective stabilizers of biological components, such as proteins and membranes, in the low-temperature or dehydrated states. This class of compounds encompasses a huge variety of possible monomeric units (differing in stereochemistry and functionalization) that can be connected in chains presenting a virtually infinite number of possible residue sequences, linkage types, and degrees of branching.
Carbohydrates (saccharides), the most abundant product of photosynthesis, play an important role in the energetic metabolism of living species and the signaling and immunological responses and are a fundamental component of the external cell wall of many organisms. In addition, saccharides are present in a variety of emerging classes of biomimetic materials. Furthermore, due to their cryo- and anhydro-protective properties, many sugars have been shown to be effective stabilizers of biological components, such as proteins and membranes, in the low-temperature or dehydrated states. This class of compounds encompasses a huge variety of possible monomeric units (differing in stereochemistry and functionalization) that can be connected in chains presenting a virtually infinite number of possible residue sequences, linkage types, and degrees of branching.
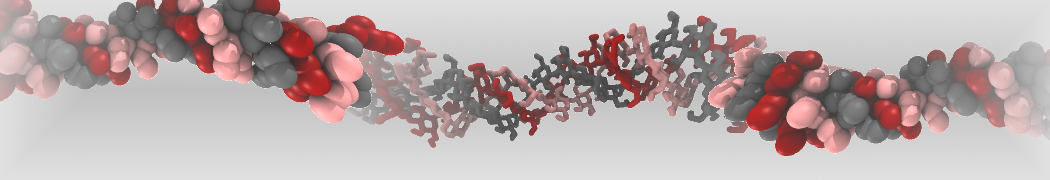
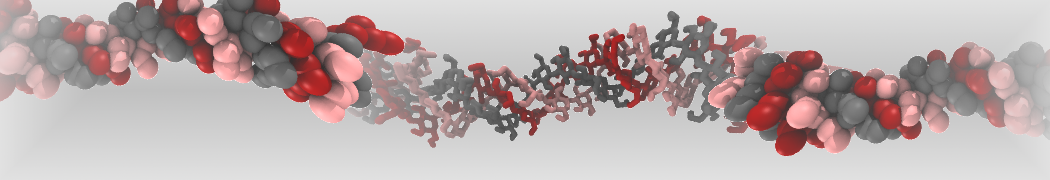
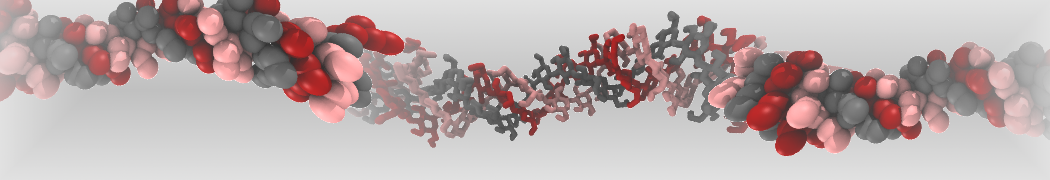
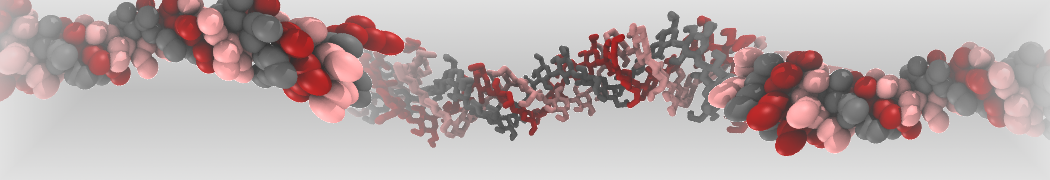
The large size of most oligosaccharides warrants the use of a coarse-grained model, yet the complexity of carbohydrate physico-chemical properties makes this a very challenging undertaking. In 2009, Martini has been parameterized for common mono- and disaccharides [1,5] that can serve as a basis for further carbohydrate modeling. Based on this model, oligosaccharides such as amylose [1], cellulose [2,6], and cyclodextrins [4] have been parameterized as well.
Other extensions include the important class of glyco-lipids, with parameters for MGDG, DGDG, SQDG, PI, PIPn, GCER, and GM1 [3], as well as lipopolysaccharides [7], and the ability to simulate glycans [8].
The latest carbohydrate parameters compatible with Martini 3 can be found here [9].