Article17
- Details
-
Last Updated: Wednesday, 21 August 2013 11:20
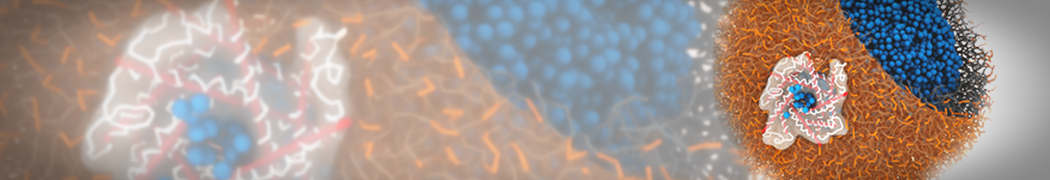
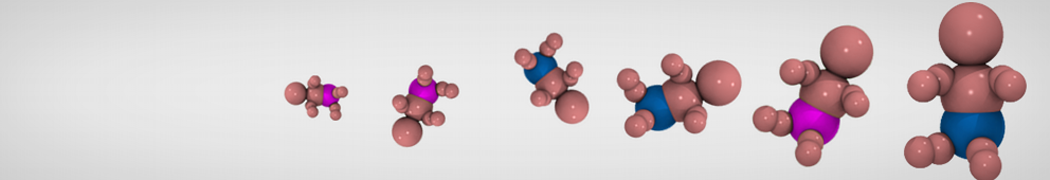
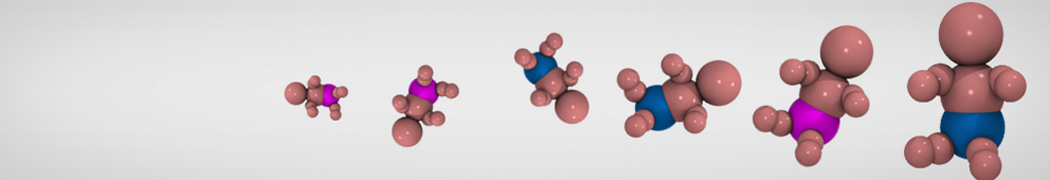
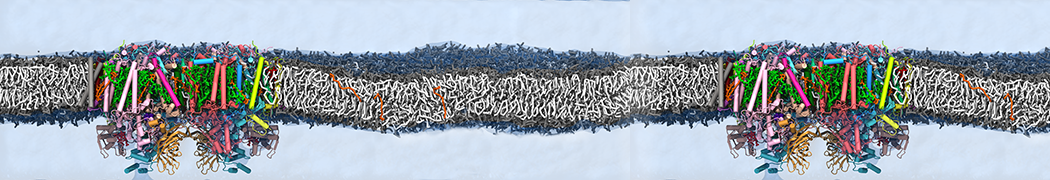
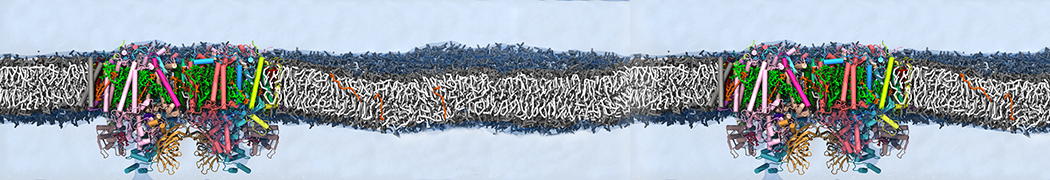
"Effects of PEGylation on the Size and Internal Structure of Dendrimers: Self-Penetration of Long PEG Chains into the Dendrimer Core" H. Lee and R.G. Larson, Macromolecules, ASAP, 2011.
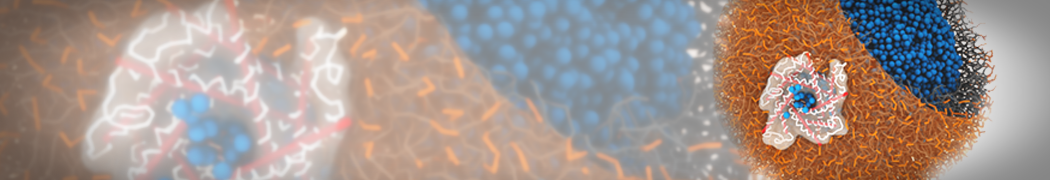
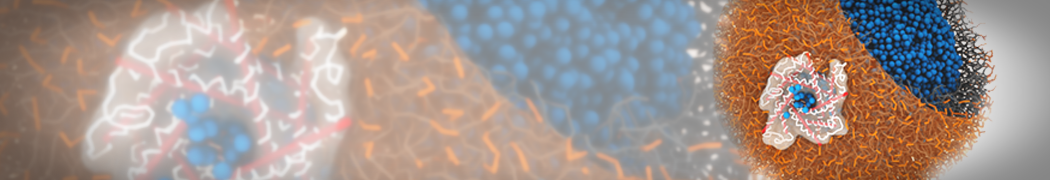
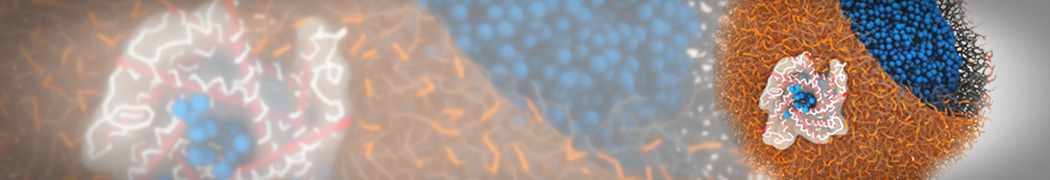
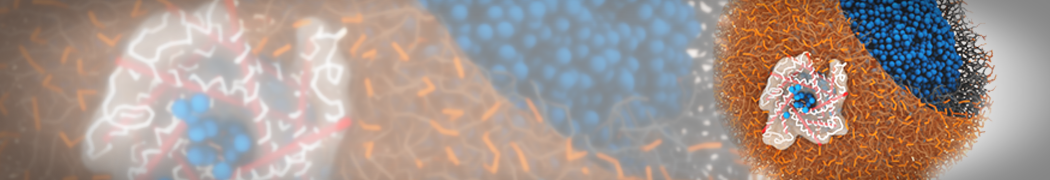
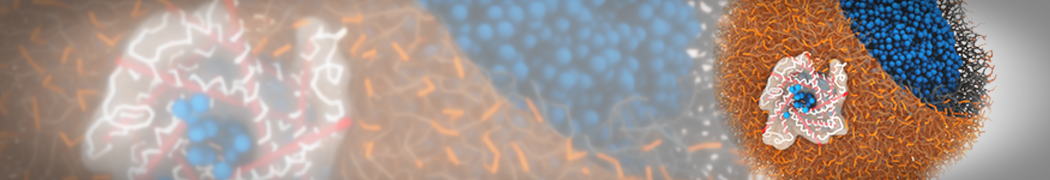
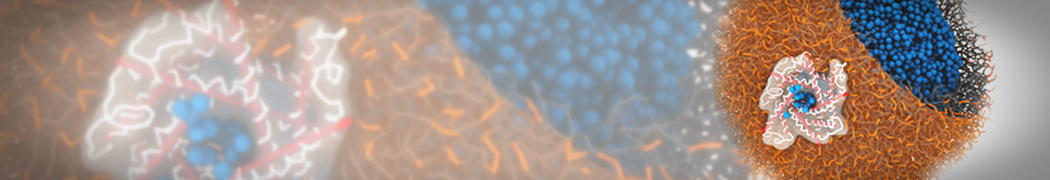
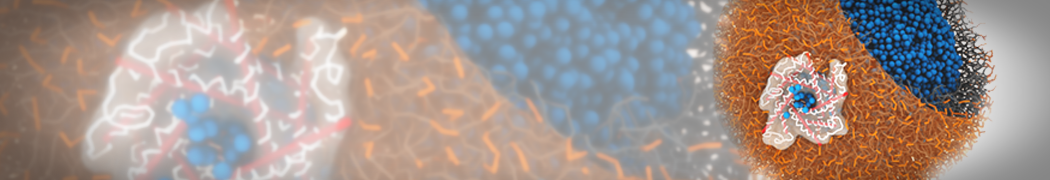
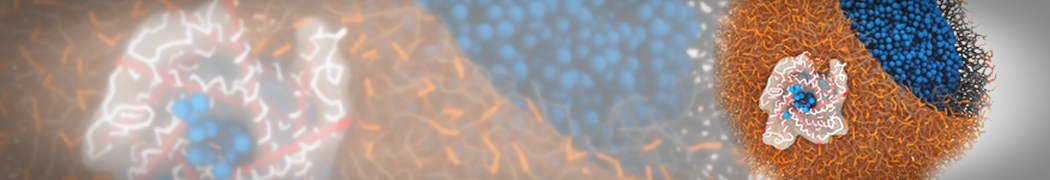
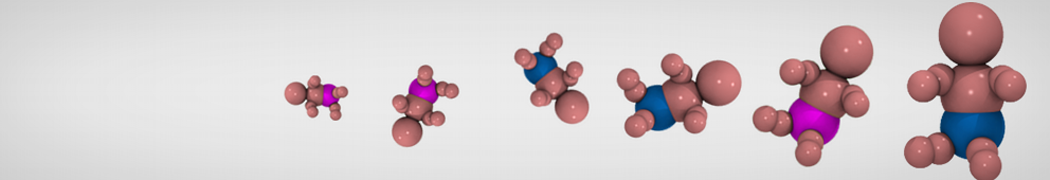
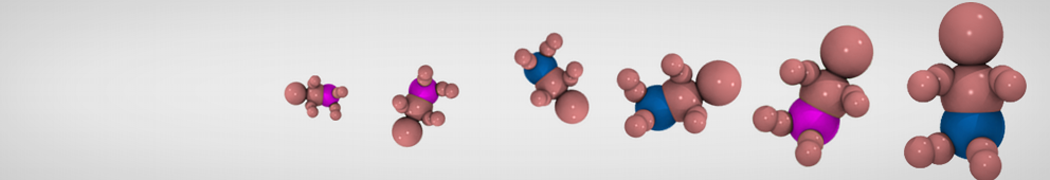
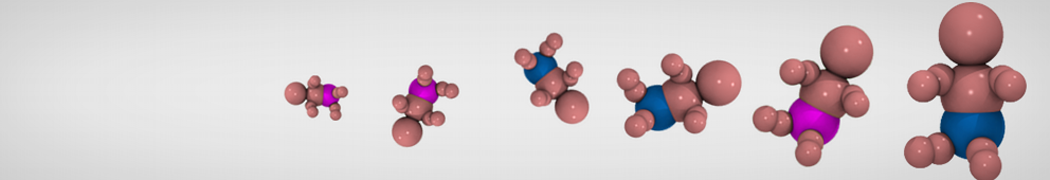
G4 PAMAM dendrimers grafted with poly(ethylene glycol) (PEG) of different sizes (Mw = 550 and 5000) and grafting densities (12−94% of surface terminals) were simulated using the coarse-grained (CG) force fields previously developed and reparametrized in this work. Simulations are carried out for G4, G5, and G7 un-PEGylated dendrimers that are either unprotonated, terminally protonated, or protonated on both terminals and interior sites, corresponding to pH values of >10, 7, and <5, respectively. As protonation increases, simulations show only a small (6% for G4 and G5) change of dendrimer radius of gyration Rg and show a structural transition from dense-core to dense-shell structure, both of which are in agreement with recent scattering experiments and all-atom simulations. For the PEGylated dendrimers, the Rg of the fully PEG(Mw = 5000)-grafted dendrimer also agrees well with experiment. Longer PEG chains with higher grafting density yield PEG−PEG crowding, which stretches dendrimer terminals toward water more strongly, leading to larger size and a dense-shell structure of the dendrimer. Long PEG chains at high grafting densities also penetrate into the dendrimer core, while short ones do not, which might help explain the reduced encapsulation of hydrophobic compounds seen experimentally in dendrimers that are 75%-grafted with long PEG’s (Mw = 5000). This reduced encapsulation for dendrimers with long grafted PEG’s has previously been attributed to PEG-induced dendrimer aggregation, but this explanation is not consistent with our previous simulations which showed no aggregation even with long PEG’s but is consistent with the new simulations reported here that show PEG penetration into the core of the dendrimer to which the PEG is attached.
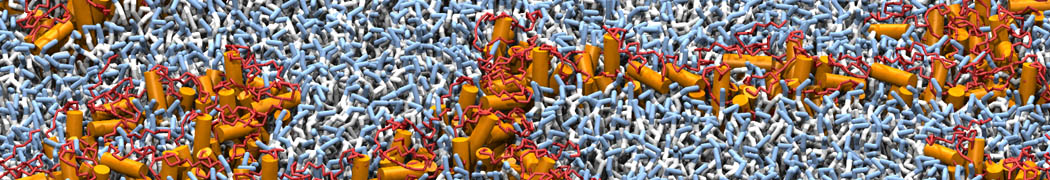
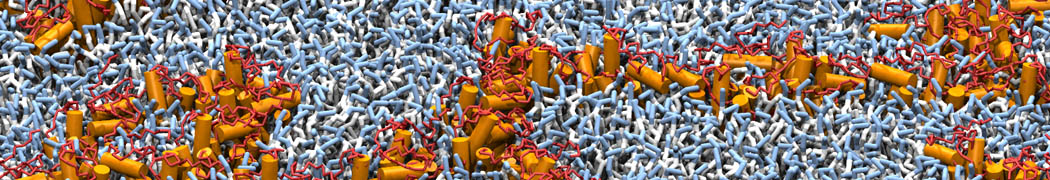
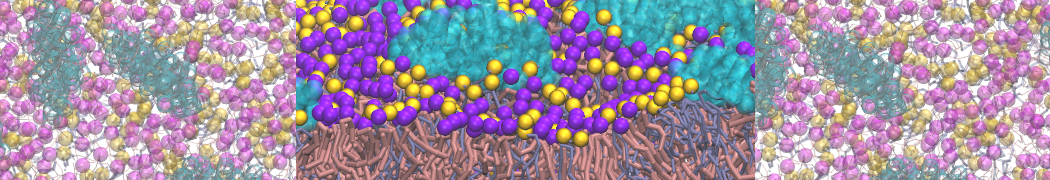
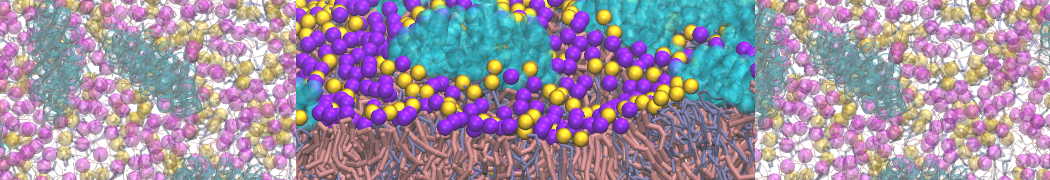
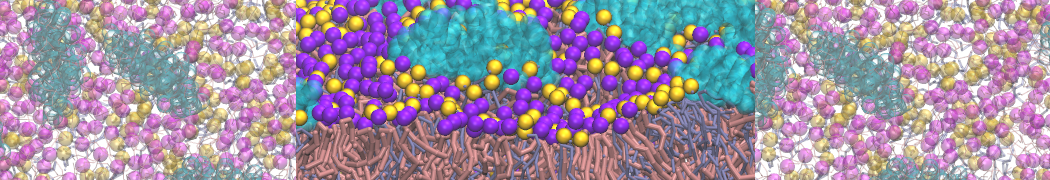
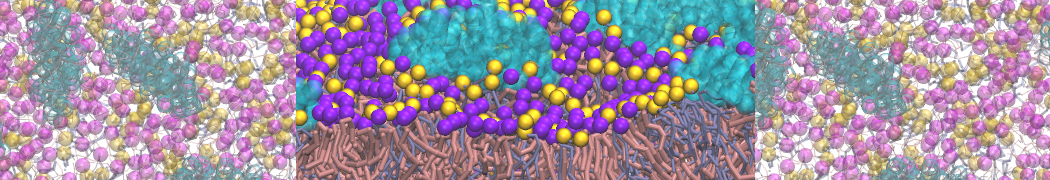
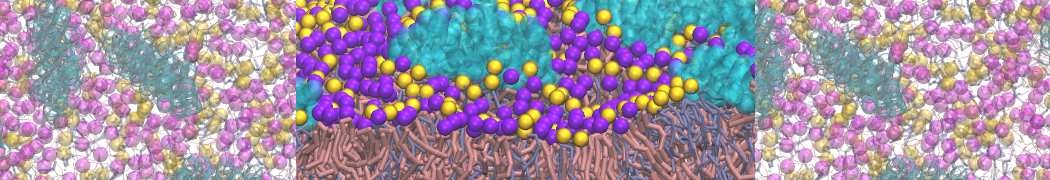
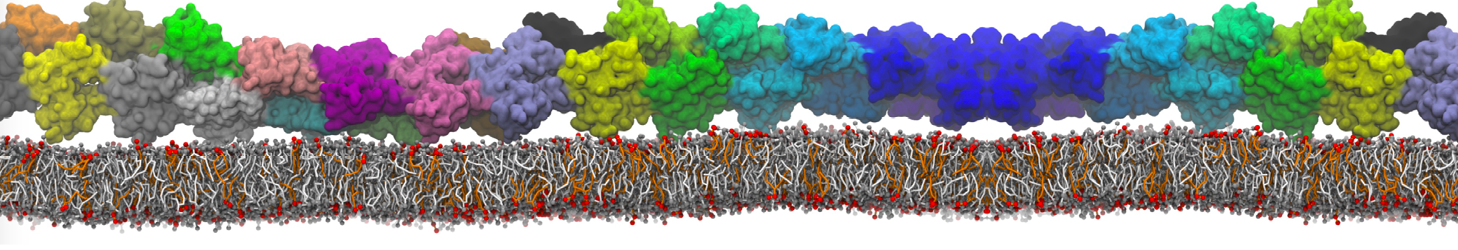
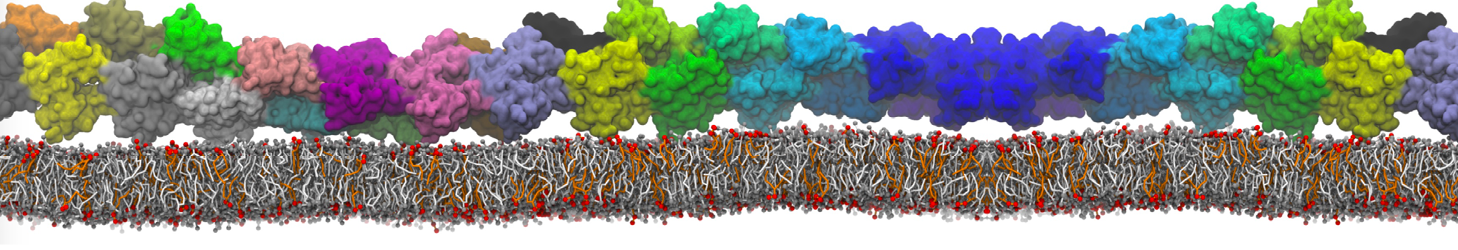
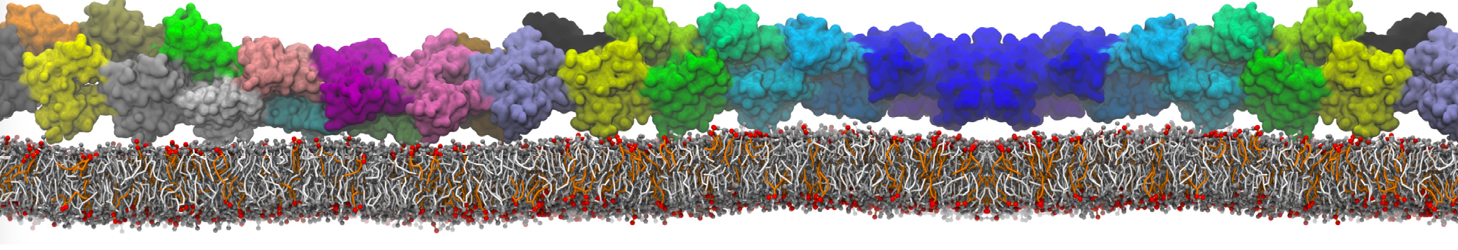
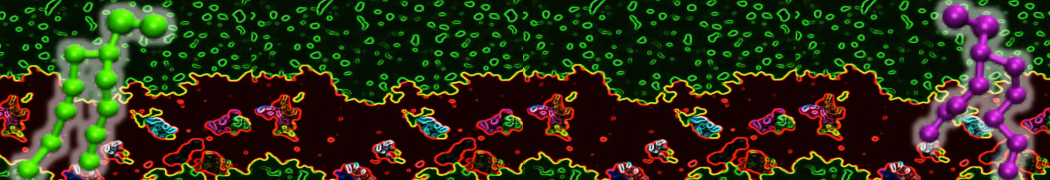
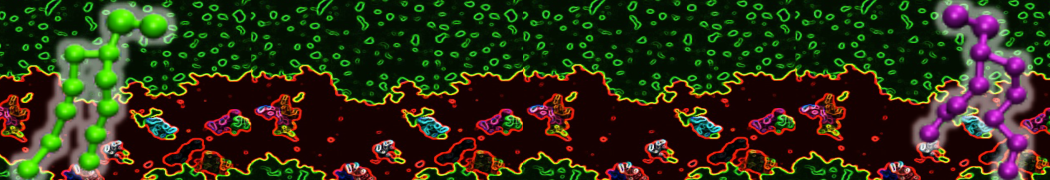
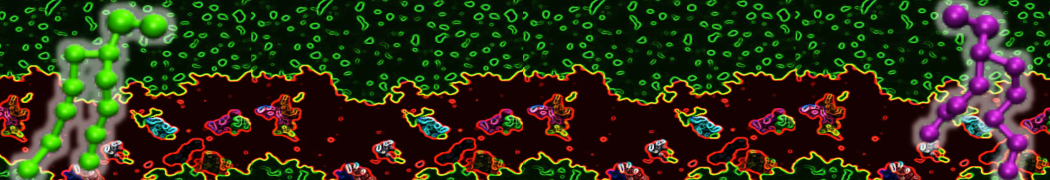
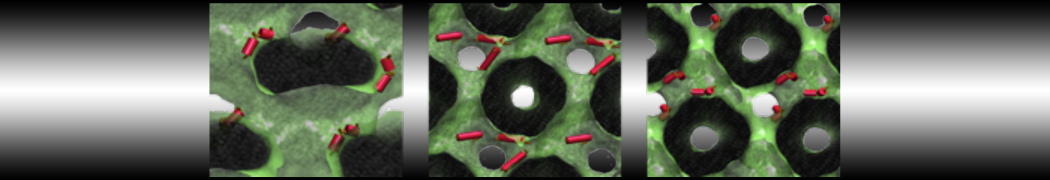
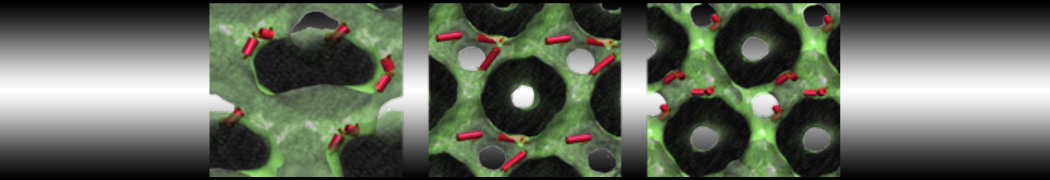
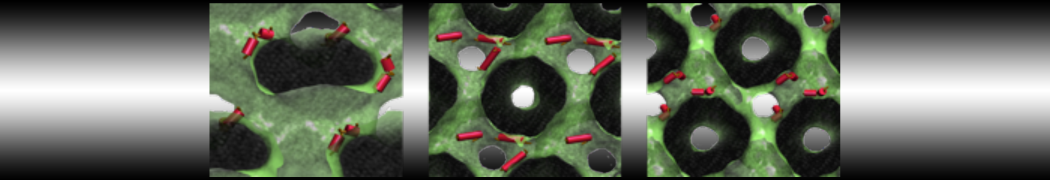
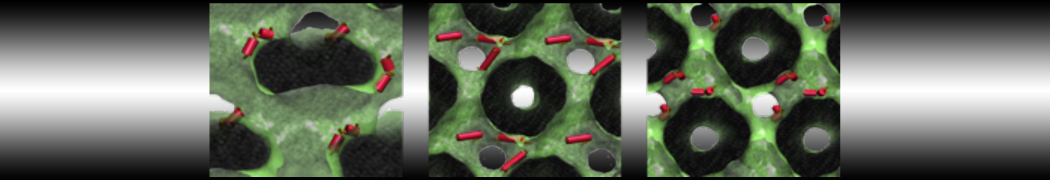
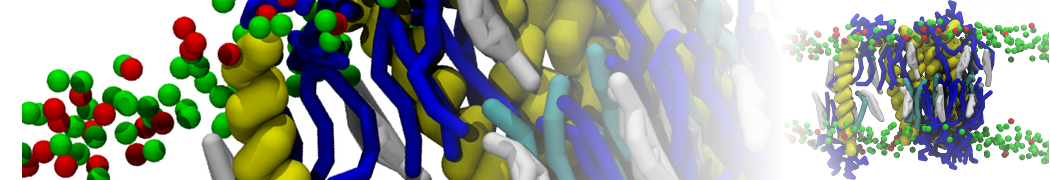
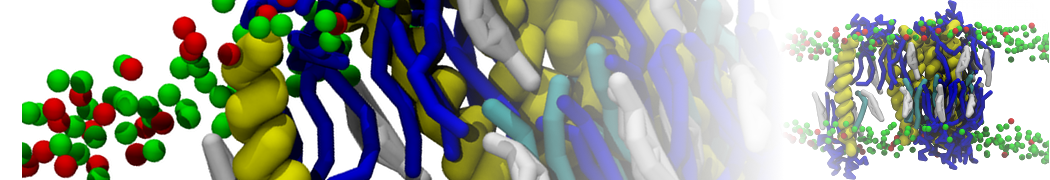
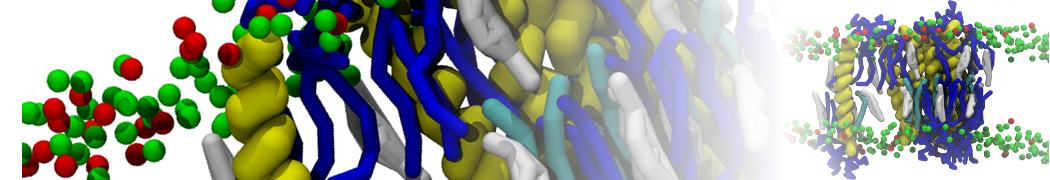
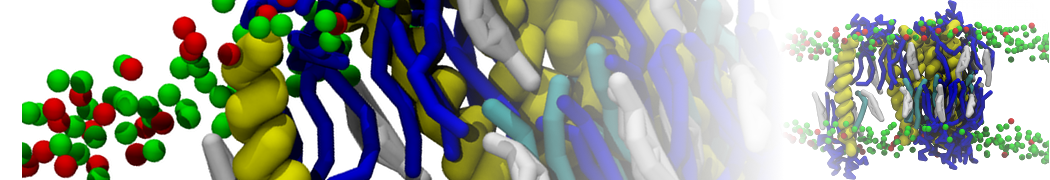
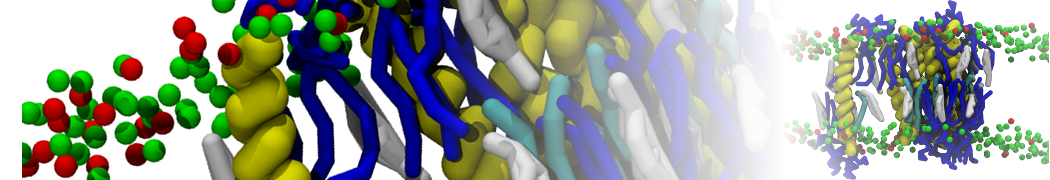
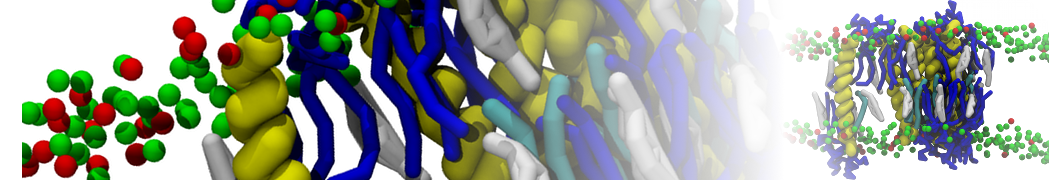
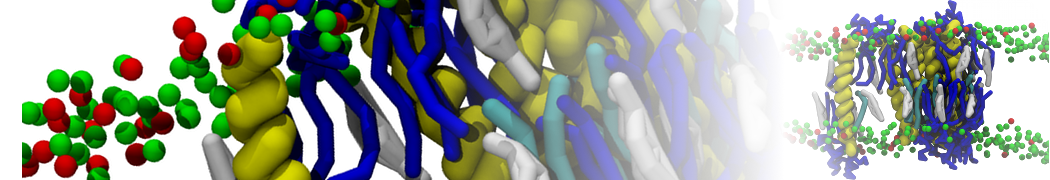
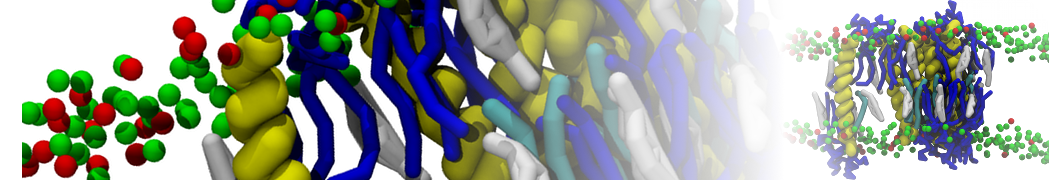
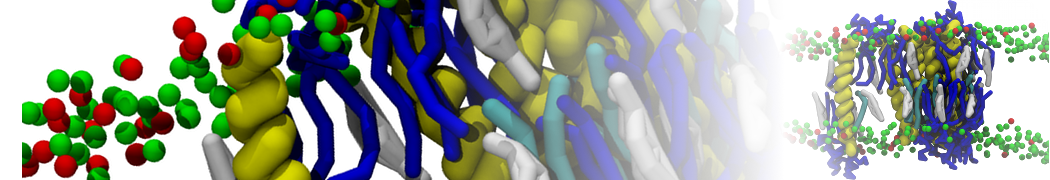
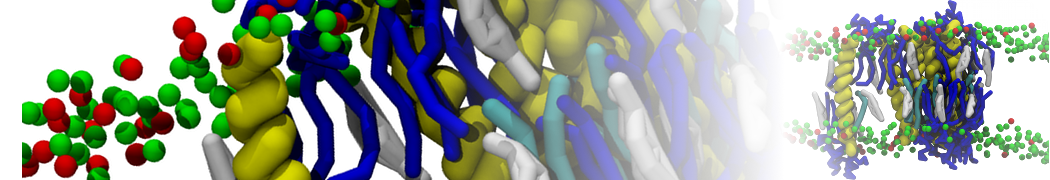
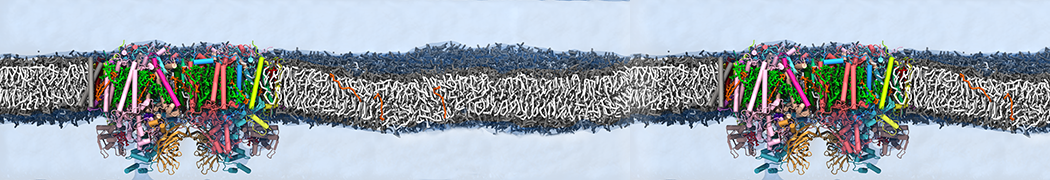
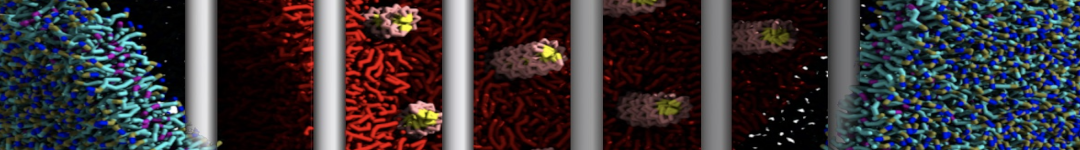
"Membrane Pore Formation Induced by Acetylated and Polyethylene Glycol-Conjugated Polyamidoamine Dendrimers" H. Lee and R.G. Larson, J. Phys. Chem. C, ASAP, 2011.
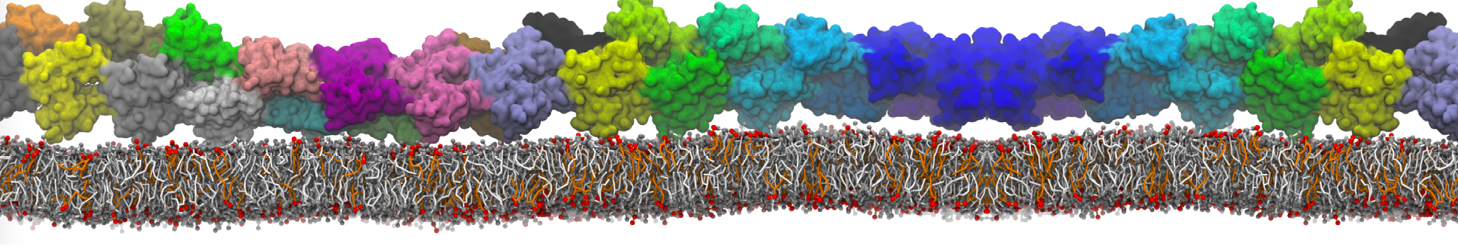
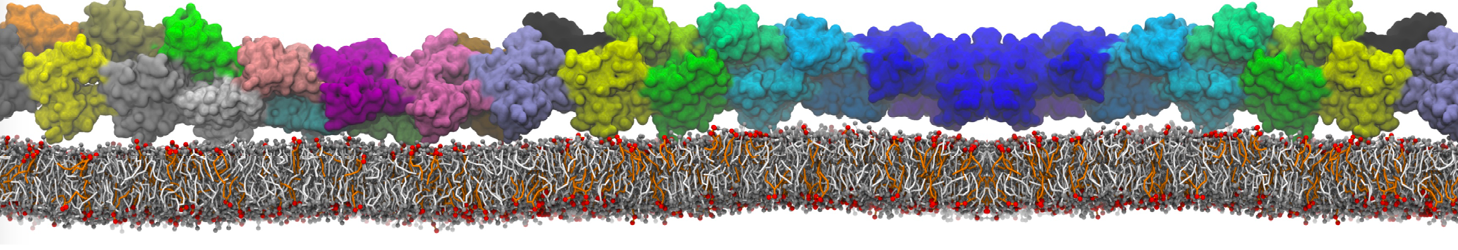
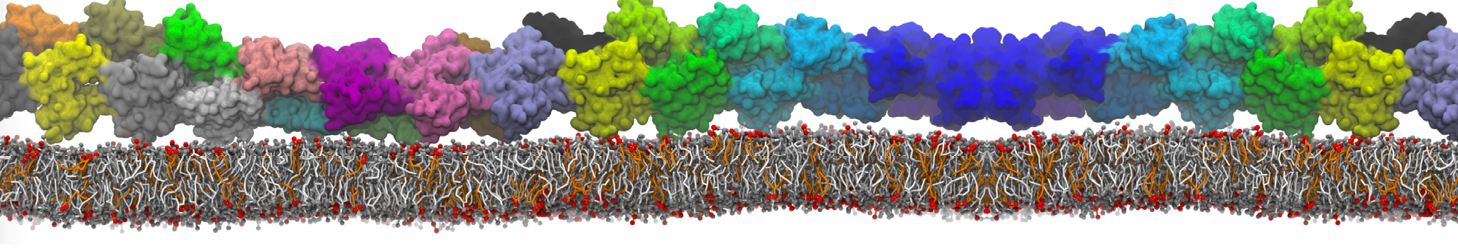
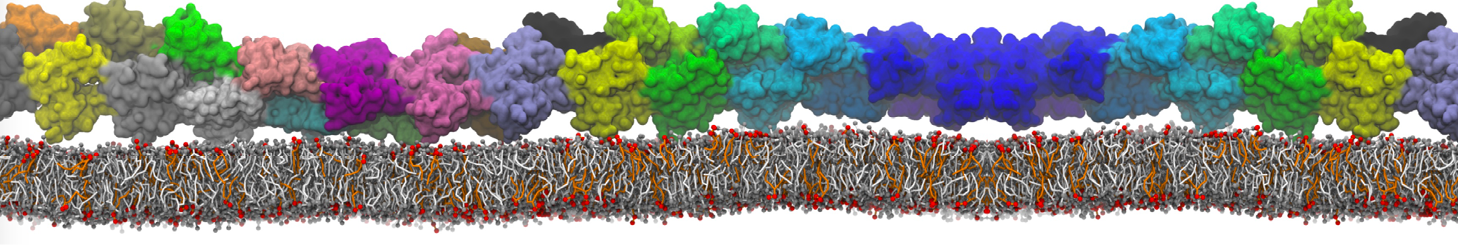
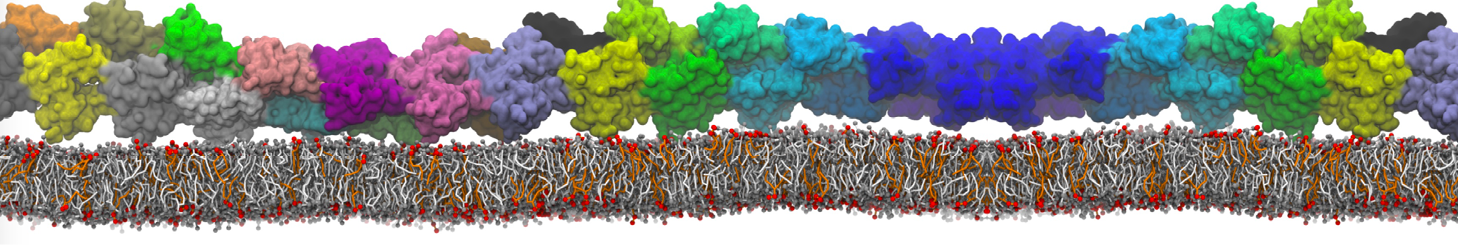
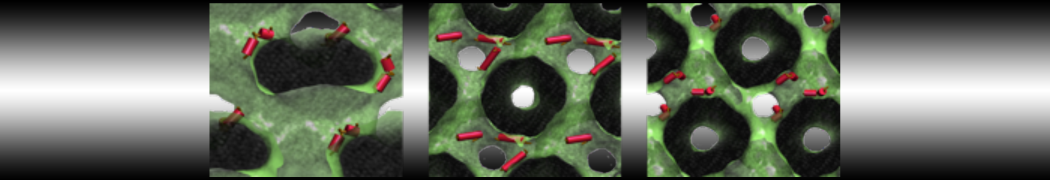
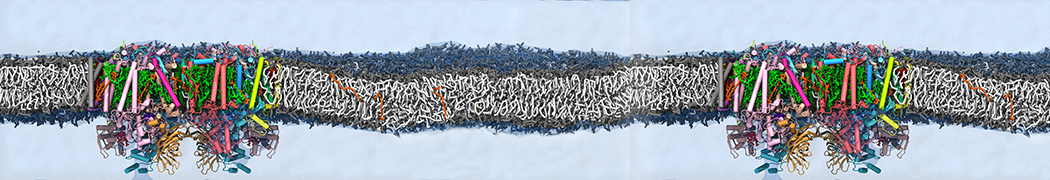
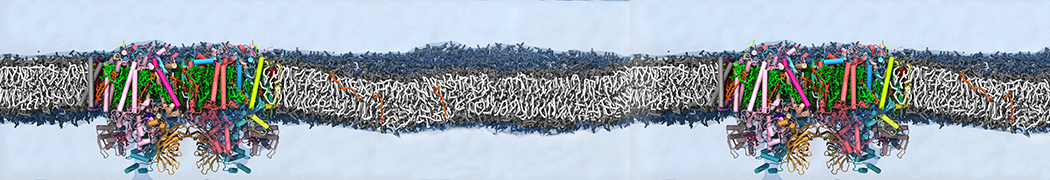
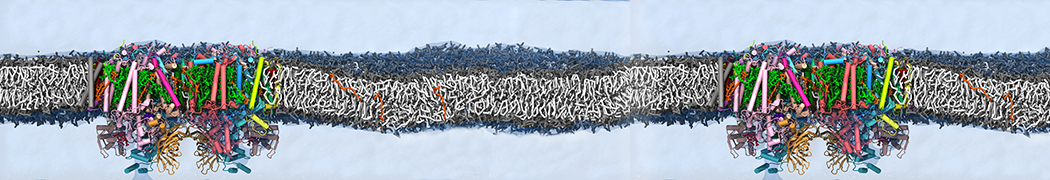
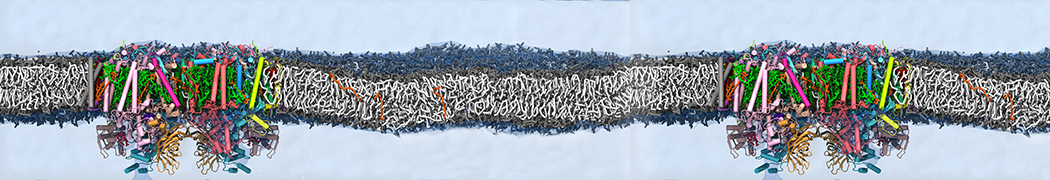
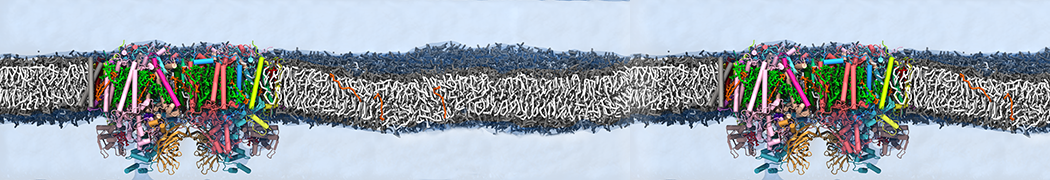
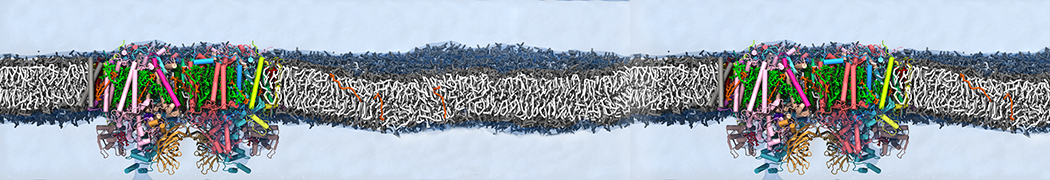
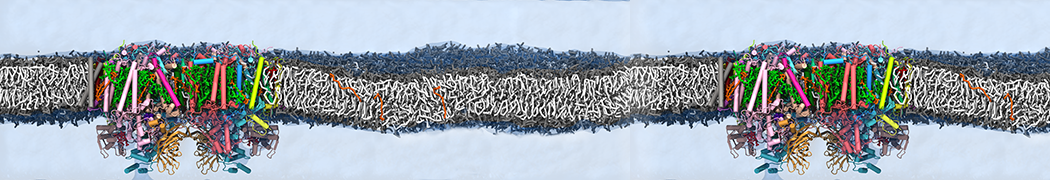
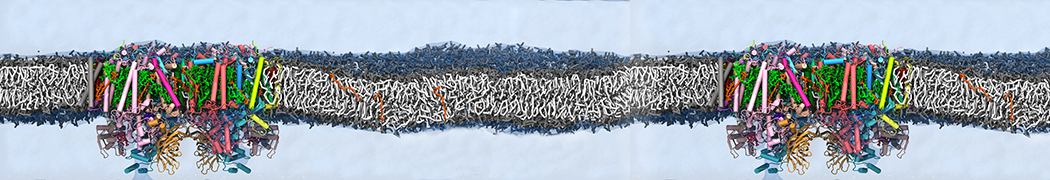
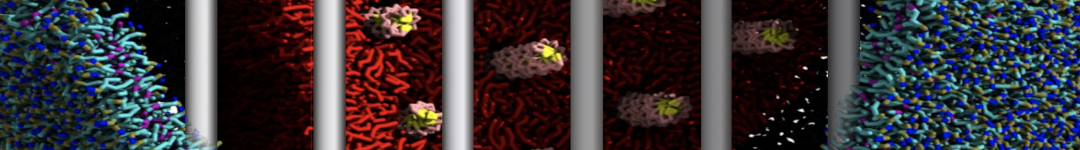
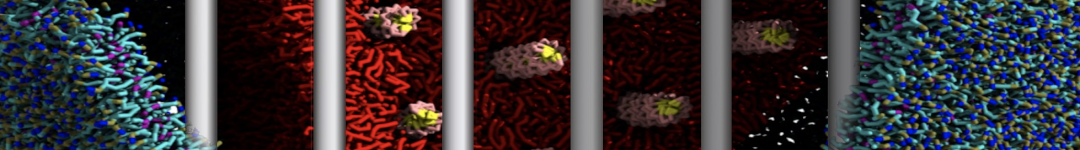
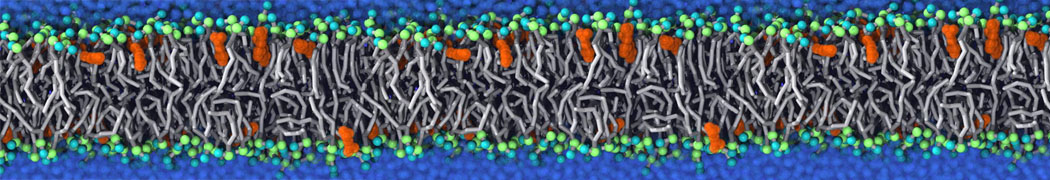
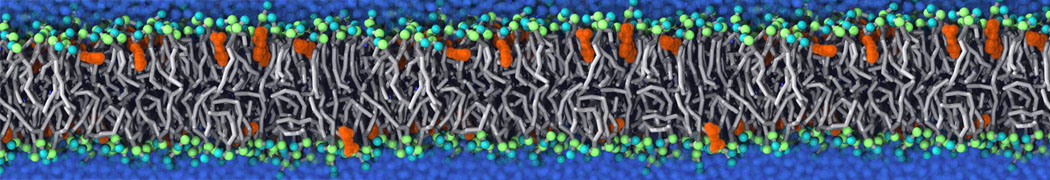
We performed molecular dynamics (MD) simulations of 36 copies of unmodified (charged), acetylated, and polyethylene glycol (PEG)-conjugated G4 dendrimers in dimyristoylphosphatidylcholine (DMPC) bilayers with explicit water using coarse-grained (CG) lipid and PEG force fields (FF). Attachment of small PEG chains to the dendrimer leads to the same reduction in membrane permeability as does attachment of acetyl groups, while a larger PEG size or a higher degree of PEGylation induces even fewer pores. This indicates that PEGylation is more efficient than acetylation in reducing membrane permeability and cytotoxicity, in qualitative agreement with experimental findings (Kim et al. Bioconjugate Chem. 2008, 19, 1660). Attachment of larger PEG chains makes the dendrimer−PEG complex larger and more spherical. Although a larger size and a more spherical shape are usually conducive to pore formation, a thick PEG layer on the dendrimer surface blocks the charge interaction between cationic dendrimer terminals and anionic lipid phosphate groups, and thus inhibits pore formation, despite the increased dendrimer size. Large PEG chains also keep the dendrimer−PEG complexes far from each other, suppressing interparticle aggregation.