Graphene nanosheets
- Details
- Last Updated: Wednesday, 21 August 2013 11:25
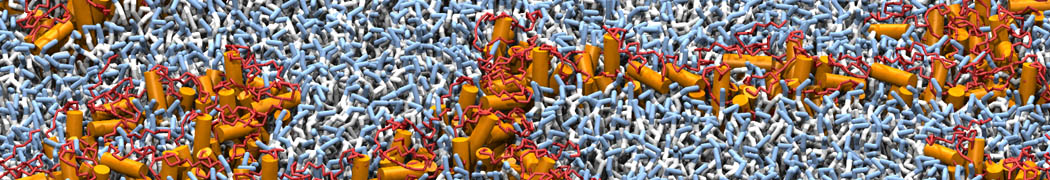
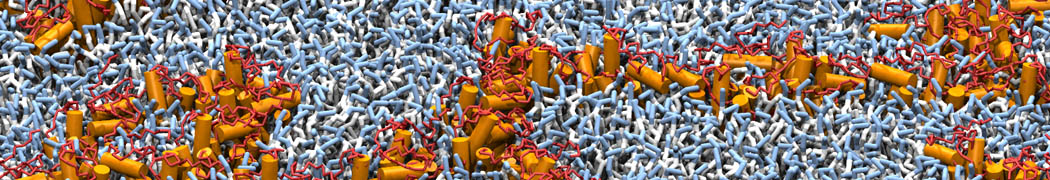
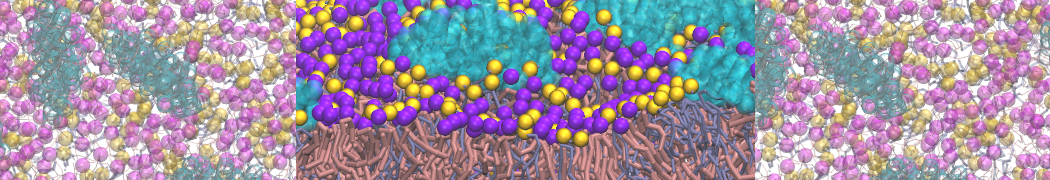
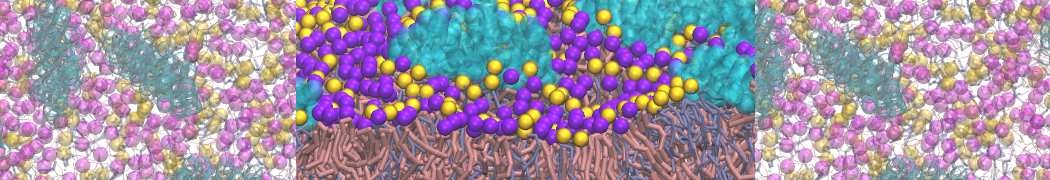
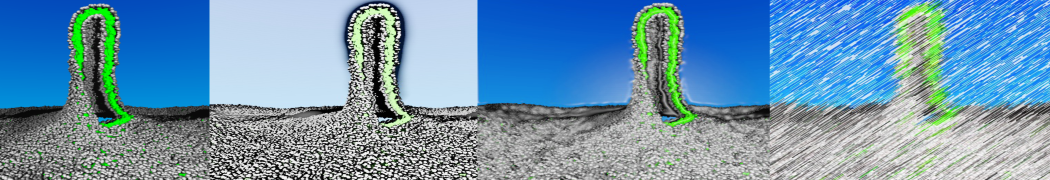
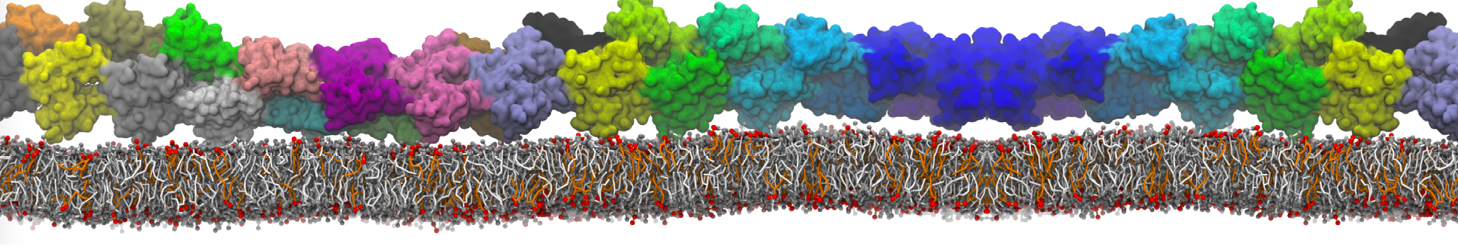
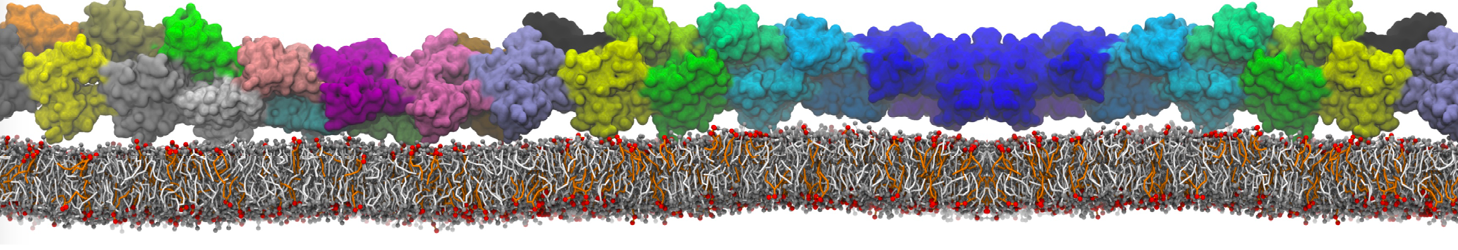
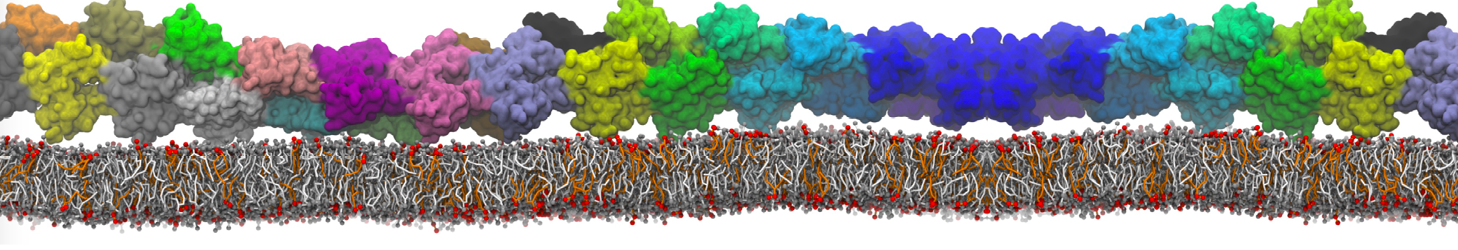
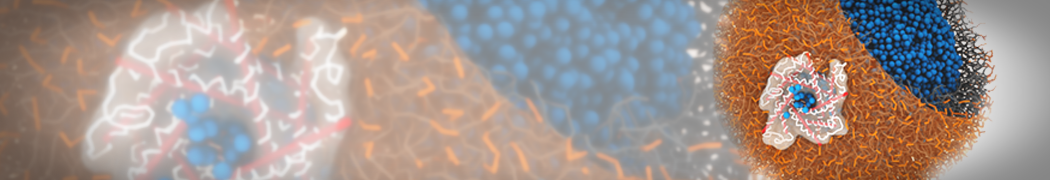
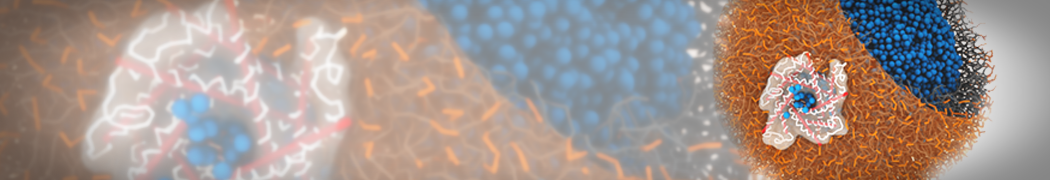
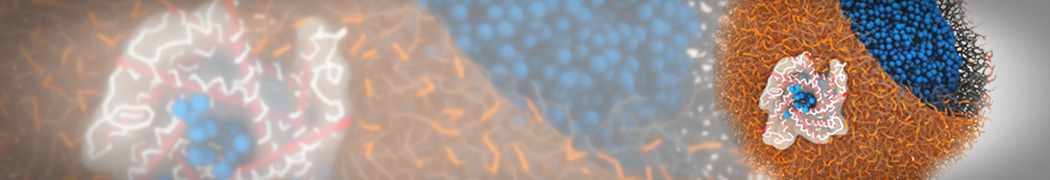
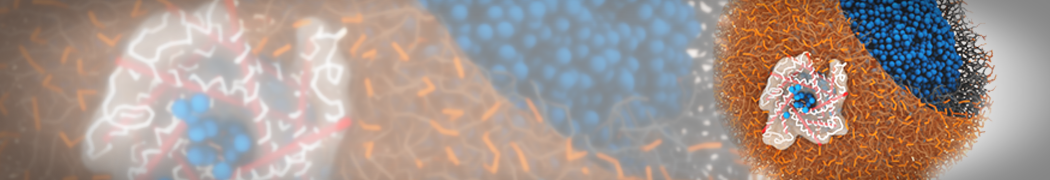
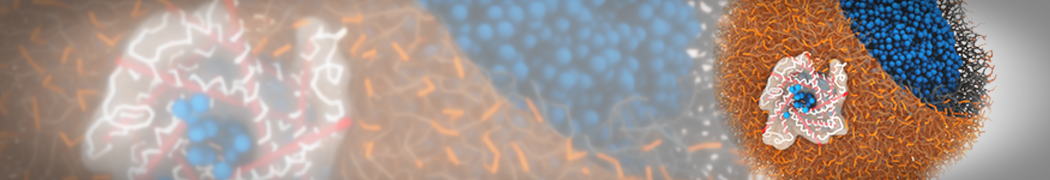
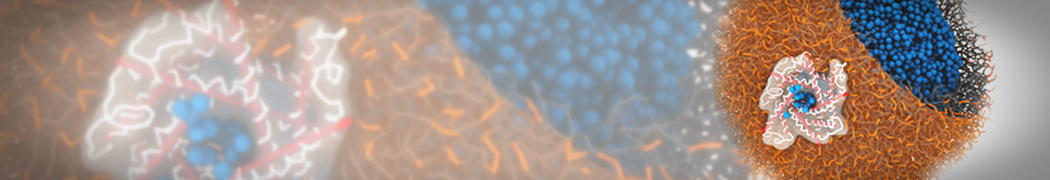
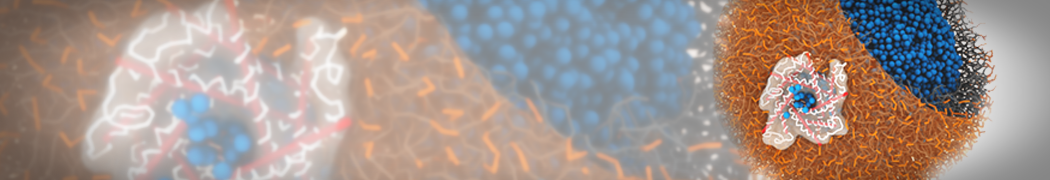
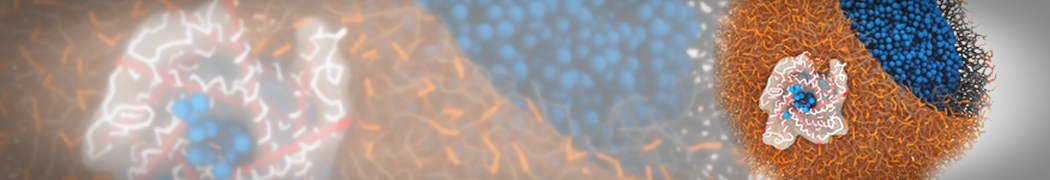
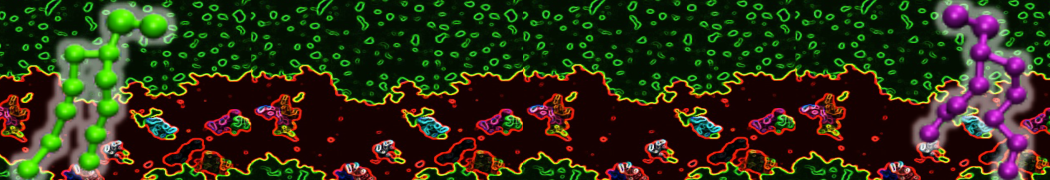
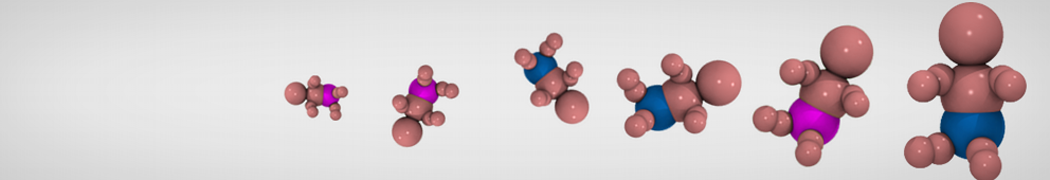
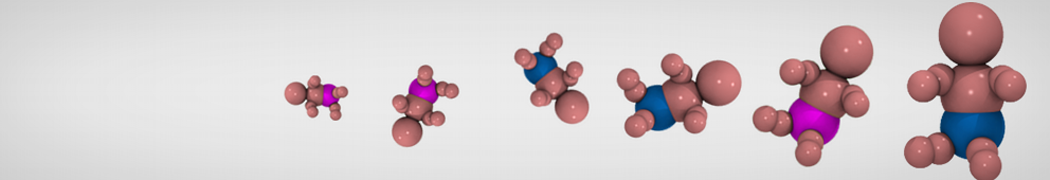
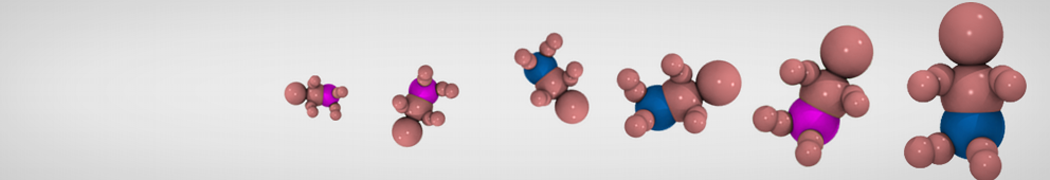
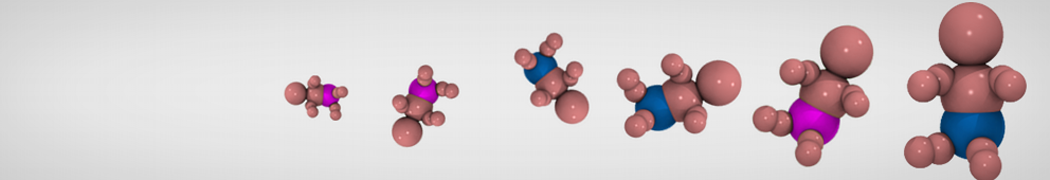
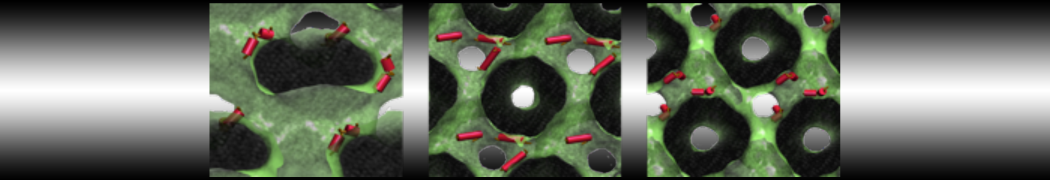
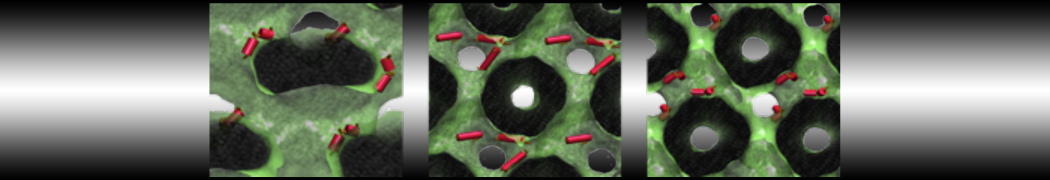
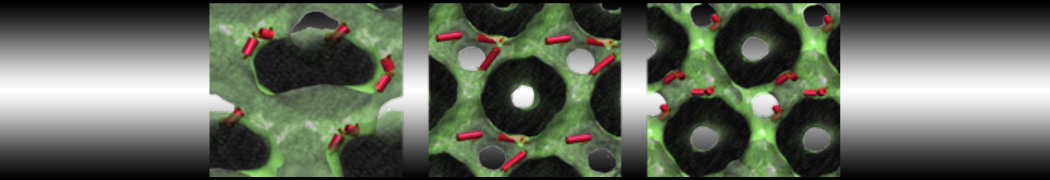
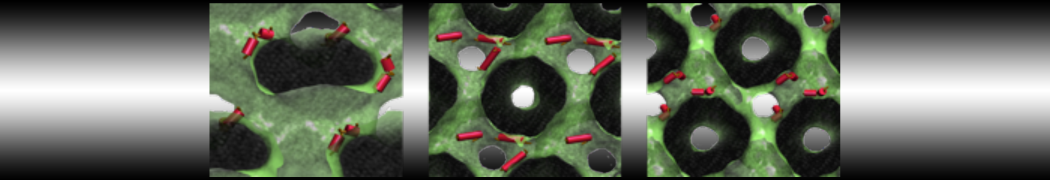
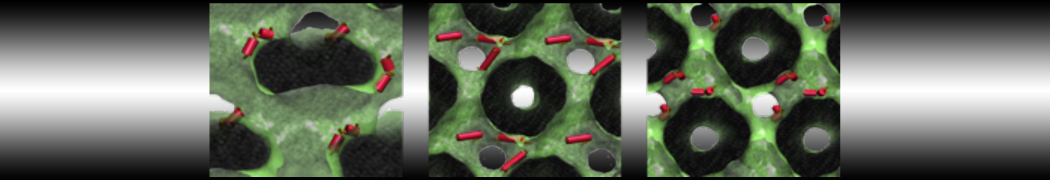
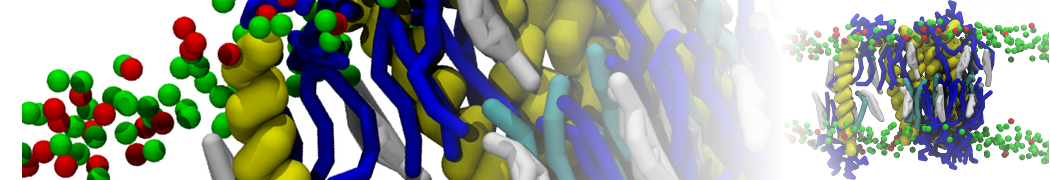
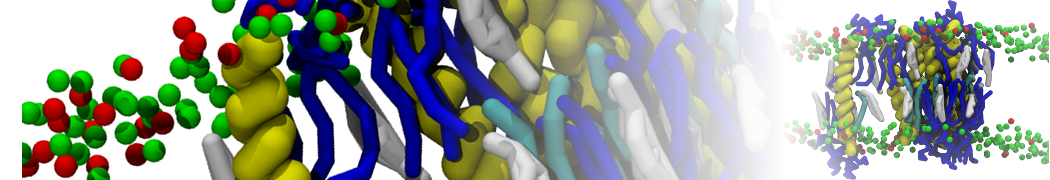
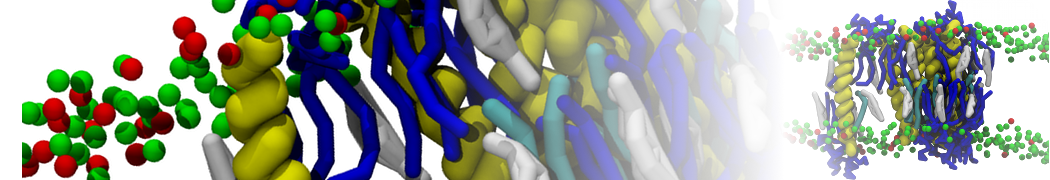
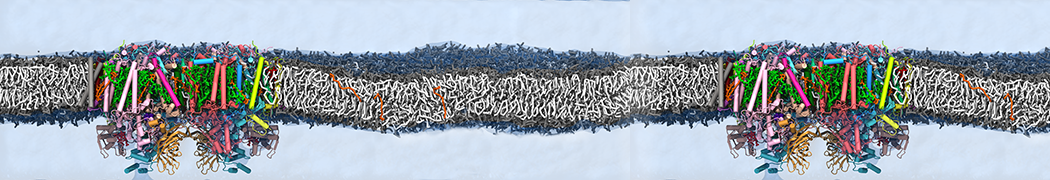
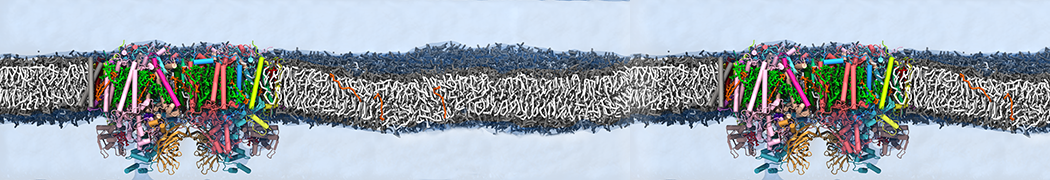
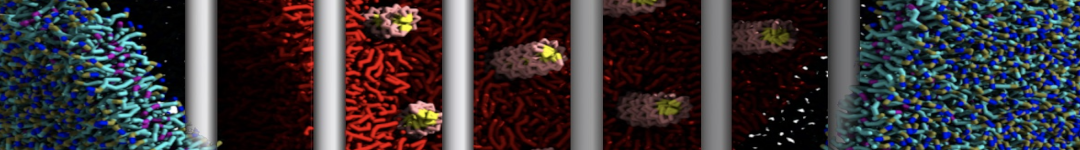
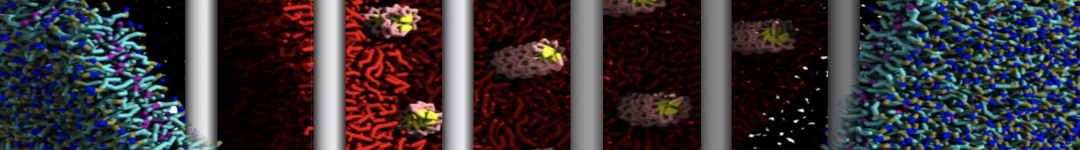
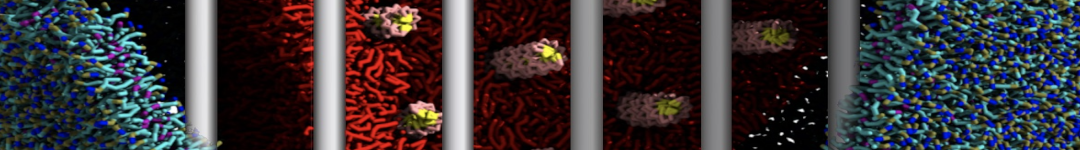
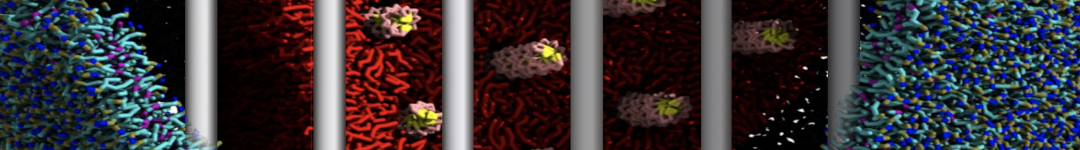
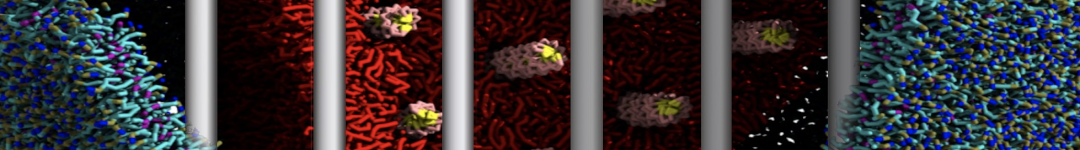
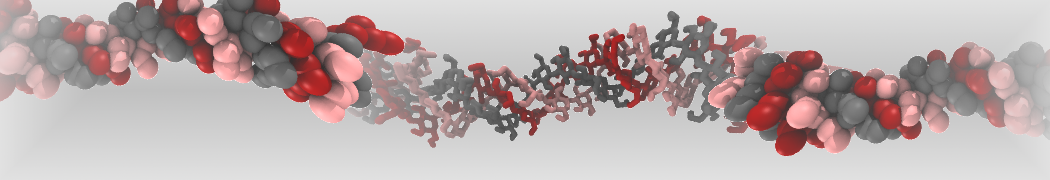
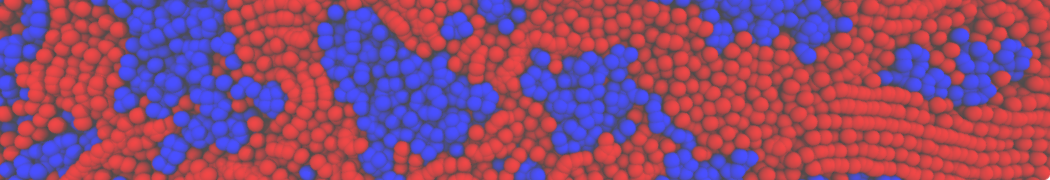
Dan Wu and Xiaoning Yang, Coarse-Grained Molecular Simulation of Self-Assembly for Nonionic Surfactants on Graphene Nanostructures. J. Phys. Chem. B, Article ASAP. DOI: 10.1021/jp3043939
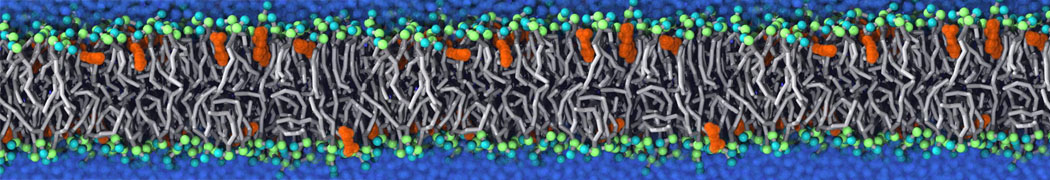
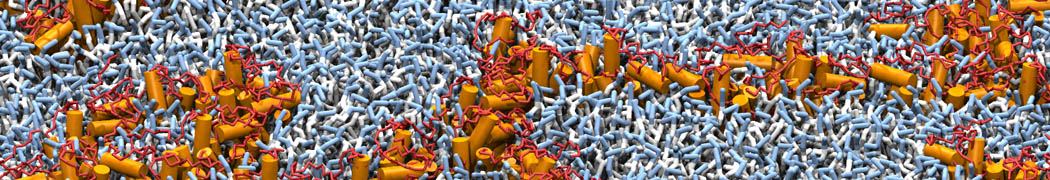
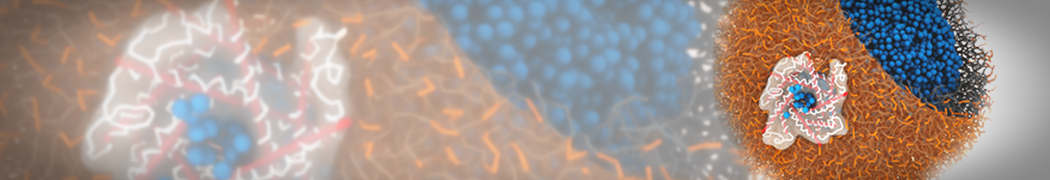
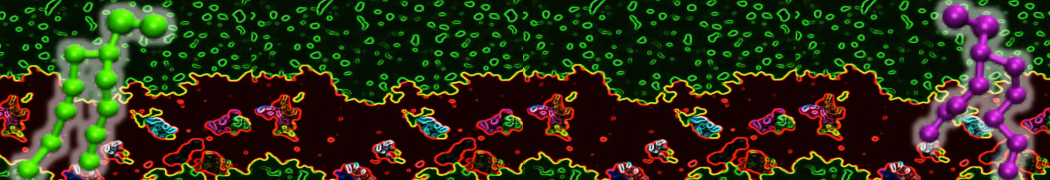
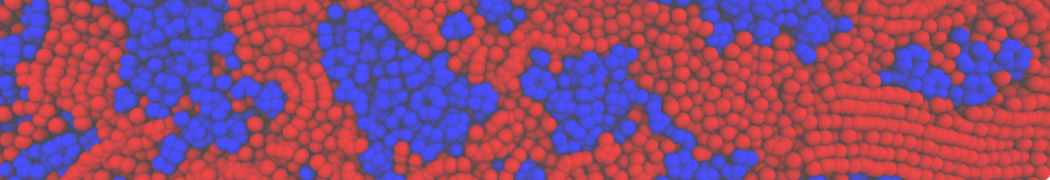
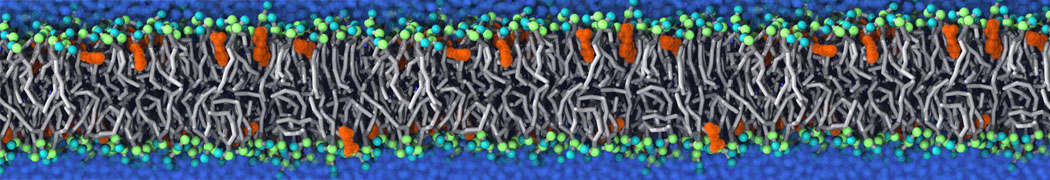
Self-assembly of amphiphilic molecules on the surfaces of nanoscale materials has an important application in a variety of nanotechnology. Here, we report a coarse-grained molecular dynamics simulation on the structure and morphology of the nonionic surfactant, n-alkyl poly(ethylene oxide) (PEO), adsorbed on planar graphene nanostructures. The effects of concentration, surfactant structure, and size of graphene sheet are explored. Because of the finite dimension effect, various morphological hemimicelles can be formed on nanoscale graphene surfaces, which is somewhat different from the self-assembly structures on infinite carbon surfaces. The aggregate morphology is highly dependent on the concentration, the chain lengths, and the size of graphene nanosheets. For the nonionic surfactant, the PEO headgroups show strong dispersion interaction with the carbon surface, leading to a side edge adsorption behavior. This simulation provides insight into the supramolecular self-assembly nanostructures and the adsorption mechanism for the nonionic surfactants aggregated on graphene nanostructures, which could be exploited to guide fabrication of graphene-based nanocomposites.